Could novel gel therapeutic set new standard of care in oncology?
Posted: 8 January 2025 | Catherine Eckford (European Pharmaceutical Review) | No comments yet
Findings from the interim clinical study showed that the gel formulation enhanced quality of life in over half of cancer patients.
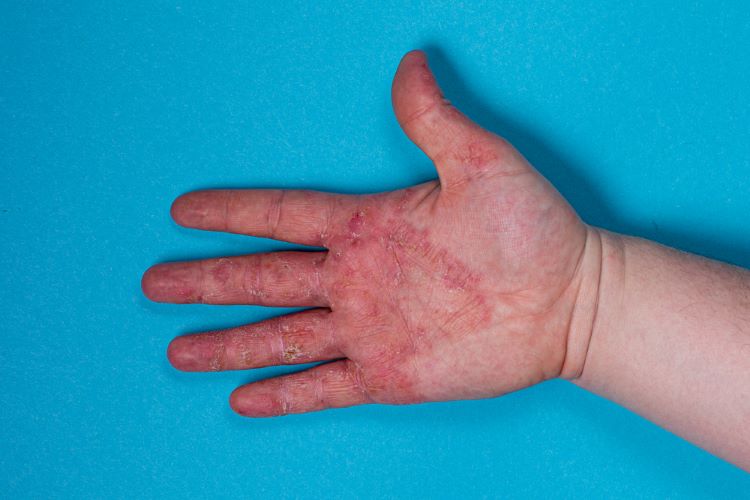

New interim results from a Phase IIa trial suggest that a gel formulation could address skin toxicities (skin, scalp, nails) associated with epidermal growth factor receptor inhibitors (EGFRi) as a cancer treatment.
What did the gel formulation trial find?
In the open-label portion of the CLEER-001 clinical trial of HT-001, 100 percent of cancer patients achieved the primary efficacy endpoint. Significant skin toxicity improvement was observed after six weeks, according to the data.
Results also showed that over fifty percent of patients (66 percent) reported reduced pain and itching scores.
Every participant was also able to maintained their full EGFRi dosage, enabling them to harness the full therapeutic effect of their EGFRi inhibitor cancer therapy”
Every participant was able to maintained their full EGFRi dosage, enabling them to harness the full therapeutic effect of their EGFRi inhibitor cancer therapy. This is notable as previous research report patients having to reduce or stop their treatment due to skin-related toxicities, Hoth Therapeutics explained.
Furthermore, no treatment-related adverse effects were reported, the company added.
Building evidence of efficiency in oncology
In Sept 2024, the company announced promising evidence for HT-001 for a first-of-a-kind treatment. Data showed that the gel formulation enabled a patient with epidermal growth factor receptor inhibitor (EGFRI) linked to papulopustular eruptions (PPEs) to discontinue Hoth Therapeutics’ therapy after one week.
“HT-001 has the potential to significantly improve the quality of life for cancer patients suffering from EGFRI-induced skin toxicities. This rapid and successful treatment is an important milestone as we continue to explore HT-001’s potential,” Robb Knie, CEO of Hoth Therapeutics, responded to the results after this data release.
Therefore, interim results from the Phase IIa study “are a significant milestone, underscoring HT-001’s potential to transform patient care by mitigating debilitating skin toxicities while maintaining critical cancer treatments. Our data highlight HT-001’s strong safety profile and the potential for it to set a new standard of care in this underserved area,” Knie commented.
Related topics
Anti-Cancer Therapeutics, Clinical Development, Clinical Trials, Data Analysis, Dosage, Drug Development, Drug Safety, Formulation, Industry Insight, Research & Development (R&D)