Preserving antibiotic efficacy with an advanced coating process to prevent Amoxicillin and Clavulanic Acid tablet degradation
Posted: 5 December 2024 | IMA Pharma | No comments yet
IMA Pharma discusses approaches to keeping Amoxicillin and Clavulanic Acid tablets stable and effective by addressing moisture sensitivity and preventing degradation.


Credit: IMA Active PERFIMA 200 - interior
Amoxicillin and Clavulanic acid are often combined in a single tablet to treat bacterial infections. The combination is designed to extend the spectrum of Amoxicillin’s antibacterial activity by including Clavulanic acid, which inhibits beta-lactamase enzymes produced by resistant bacteria. However, these components have stability and formulation challenges, especially concerning moisture sensitivity, which directly impacts their coating requirements and overall stability.
Amoxicillin is a semi-synthetic antibiotic that belongs to the penicillin class. It is a beta-lactam antibiotic, which makes it sensitive to hydrolysis, especially in the presence of moisture. Hydrolysis can break down the beta-lactam ring, rendering the antibiotic ineffective.
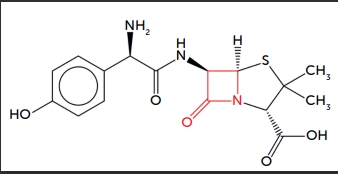

Figure 1: Molecular structure of Amoxicillin together with its antibacterial properties from the existence of beta-lactam ring (in red).
Amoxicillin is also moderately hygroscopic, meaning it tends to absorb moisture from the environment. The presence of moisture can lead to degradation of Amoxicillin, reducing its potency and shelf-life. The primary degradation pathway for Amoxicillin in the presence of moisture is hydrolysis.
Clavulanic acid is a beta-lactamase inhibitor and is structurally like penicillin. It prevents the degradation of beta-lactam antibiotics (like Amoxicillin) by inhibiting the enzymes that bacteria produce to resist them. It is also highly hygroscopic and very sensitive to moisture. It is more prone to degradation compared to Amoxicillin, especially when exposed to moisture, light, and heat. This is primarily due to its beta-lactam ring, which can be easily hydrolysed. Exposure to moisture can cause Clavulanic acid to degrade rapidly into inactive compounds, which diminishes the effectiveness of the drug combination.
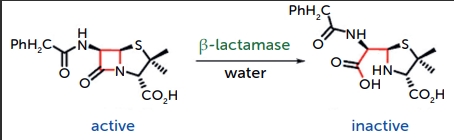

Figure 2: example of beta-lactam ring degradation via hydrolysis
Challenges in preventing tablet degradation
Given their moisture sensitivity, both Amoxicillin and Clavulanic acid tablets require a robust coating to protect the active pharmaceutical ingredients (APIs) from environmental factors.
Common materials for this purpose include polymers like hydroxypropyl methylcellulose (HPMC), ethyl cellulose (EC), and polyvinyl alcohol (PVA). These polymers form a thin, protective layer around the tablet core. To further enhance moisture protection, additional substances such as plasticisers (eg, polyethylene glycol or PEG) and moisture-barrier agents (eg, polyvinyl alcohol) may be included in the coating formula.
If not properly coated and packaged, Amoxicillin and Clavulanic acid tablets can degrade, leading to a loss of potency and therapeutic efficacy. The shelf-life of the product is directly related to how well it is protected from environmental factors, particularly moisture.


Image: IMA Active PERFIMA 200
Achieving optimal moisture protection
The coating process for these tablets is usually performed in a controlled environment to minimise moisture exposure. Humidity control is critical during manufacturing and packaging.
The coating must be applied evenly and at an appropriate thickness (normally at least a weight gain of four percent) to provide adequate protection while maintaining the desired dissolution profile.
Given the moisture sensitivity of both components, especially Clavulanic acid, desiccants (such as silica gel packs) are often included in the packaging to control humidity levels within the container.
Tablets may be individually blister-packed in aluminium/aluminium (Alu/Alu) blisters, which provide superior moisture protection compared to standard PVC blister packs.
As Amoxicillin and Clavulanic acid are sensitive to moisture, the dehumidified and temperature-controlled air ensures that the active ingredients do not degrade during the coating process, thereby maintaining the potency and effectiveness of the drug.
An inlet air treatment unit is a system used to condition the air that enters a coating machine, ensuring it meets specific requirements for temperature, humidity, and cleanliness. For pharmaceutical coating processes, particularly for moisture-sensitive products, the primary goal is to control the relative humidity (RH) and temperature of the air to ensure consistent coating quality and protect the active ingredients.
To maintain the quality and stability of moisture-sensitive tablets like Amoxicillin and Clavulanic acid during the coating process, the inlet air treatment unit with silica gel rotor dehumidification technology and high accuracy of the coater control system regulation are crucial. By providing precise control over humidity and temperature, the system ensures that the coating environment is ideal, protecting the active ingredients and resulting in a consistent, high-quality product.
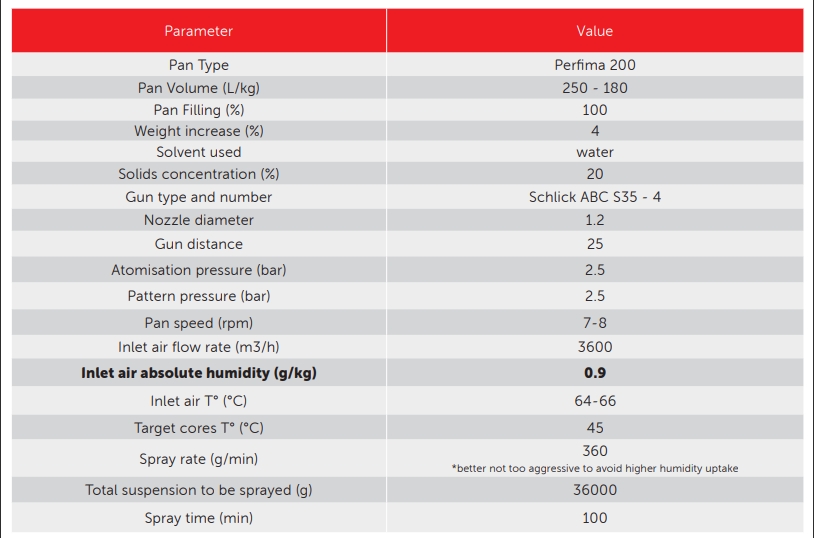

Table 1: formulation and process conditions for Amoxicillin-based tablet coating using an Opadry AMB II formulation.6
the inlet air used provides an absolute humidity value of 0.9 percent that is incredibly low compared to standard processes”
One example of formulation and process conditions is reported below for coating in a perforated drum using an Opadry AMB II providing a 20 percent concentration in water for a four percent weight gain.
It can be noted that the inlet air used provides an absolute humidity value of 0.9 percent that is incredibly low compared to standard processes.
In summary, to ensure the stability and efficacy of Amoxicillin and Clavulanic acid tablets, coatings are designed to provide a strong barrier against moisture and other environmental factors. The choice of coating materials and packaging solutions is crucial to protecting these moisture-sensitive drugs, ensuring their shelf-life, and maintaining their therapeutic effectiveness. Proper manufacturing practices, including humidity and temperature control, further contribute to the stability of the finished product.
About the author
Caterina Funaro is IMA Active Competence Center Manager and Coating Expert.
References
1. Aulton, M E, Taylor, K M G. Aulton’s Pharmaceutics: The Design and Manufacture of Medicines (5th ed.). 2018. Elsevier.
2. Lachman L, Lieberman H A, Kanig J L. The Theory and Practice of Industrial Pharmacy (4th ed.). 2009. CBS Publishers & Distributors.
3. Rowe R C, Sheskey P J, Quinn M E. Handbook of Pharmaceutical Excipients (6th ed.). 2009. Pharmaceutical Press.
4. Qiu Y, Chen Y, Zhang G G Z, Yu L, Mantri R V. Developing Solid Oral Dosage Forms: Pharmaceutical Theory and Practice (2nd ed.). 2016. Academic Press.
5. Fiese E F, Hagen T A. Preformulation. In G. S. Banker & C. T. Rhodes (Eds.), Modern Pharmaceutics (3rd ed.). 1998. CRC Press.
6. Opadry® amb II High Performance Moisture Barrier Film Coating. [Internet] Colorcon. Available from: https://www.colorcon.com/markets/pharmaceuticals/film-coatings/immediate-release/opadry-amb-ii
Related topics
Antibiotics, Biopharmaceuticals, Formulation, Manufacturing, Packaging, Production, QA/QC, Technology, Therapeutics









