Preventing fungal contamination in pharmaceuticals
Posted: 11 March 2024 | Catherine Eckford (European Pharmaceutical Review) | No comments yet
While fungal contamination detection in pharmaceutical manufacturing remains a challenge, preventive practices and policies must be followed, research states.
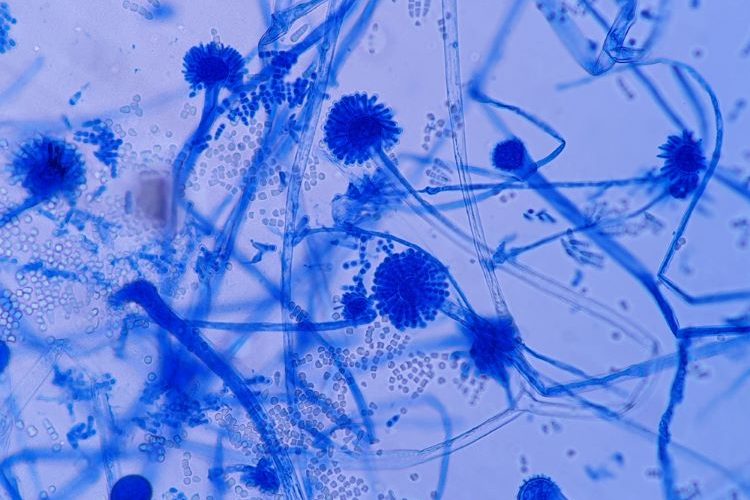

In a review on fungal-contaminated compounded pharmaceuticals and medical devices, researchers have described how the contamination of these products can be due to breaches in sterile compounding procedures.
The authors explained: “inadequate sterile compounding techniques or storage conditions, or exceeding the limit of a fungal count, can result in fungal contamination.”
Additionally, this can be caused by “poor handling, repackaging, and nonadherence to good manufacturing practices [GMP] during dispensing and packaging have resulted in the microbiological contamination of non-sterile pharmaceutical products.”
Employing MALDI-TOF MS for microbial identification in sterile drug manufacturing
Fungal contamination in pharmaceutical cleaning facilities
Case study examples
Mould and yeast have been reported by several previous authors in numerous pharmaceutical cleanrooms, cold rooms, and controlled areas, according to the authors. For example, rising ambient temperatures and ingress of mould from items entering cleanrooms, has resulted in some vaccine and pharmaceutical companies in Europe experiencing increased contamination.
Ahmed et al. elaborated, noting that based on recall data collected by the US Food and Drug Administration (FDA) in over 100 products between 2000–2010, “fungi existed in 21 percent of products.” The authors also shared: “in the last three years [since 2023], the US FDA has recalled several drugs due to fungal contamination.”
One case of drug contamination from 2021 was highlighted in the paper. The authors stated that the presence of Aspergillus penicillioides in antimicrobial preoperative skin preparation drugs were attributed to inadequate storage conditions.
An earlier case of contamination from 2013 of manufactured steroid injections containing methylprednisolone acetate was also mentioned in the paper. According to the authors, these were linked to 55 fatal cases of fungal meningitis.
Preventing contaminated drug products
The authors wrote that because fungal identification is challenging, “the examination of fungi by official authorities for microbial quality control is limited.” In the paper, several approaches to diagnosing fungal species were highlighted, including Infrared Fourier transform, Surface-Enhanced Raman Scattering, Solid-Phase Cytometry, Nuclear Magnetic Resonance, biosensors and nanotechnology.
According to Ahmed et al., “nanotechnologies may help to improve current fungal diagnostic practices, as accurate and rapid detection of fungi is often necessary to determine the appropriate corrective actions.”
In summary, considering the serious diseases caused by contaminated pharmaceuticals and the challenges discussed, Ahmed et al. asserted: “While performing efficient disinfection and antiseptic operations, only goods that adhere to the verified methodologies described in normative standards should be utilised.”
The paper was published in Current Microbiology.
Related topics
Cleanrooms, Drug Manufacturing, Drug Safety, Environmental Monitoring, Impurities, Industry Insight, Lab Equipment, Manufacturing, Medical Devices, Microbial Detection, Microbiology, Production, QA/QC, Therapeutics









