Boehringer Ingelheim to present highly anticipated data from landmark TIOSPIR™ trial at ERS 2013
Posted: 2 September 2013 | | No comments yet
Results from TIOSPIR™ will be presented for the first time at the Annual Congress of the European Respiratory Society…


Results from TIOSPIR™ (Tiotropium Safety and Performance in Respimat), one of the largest global chronic obstructive pulmonary disease (COPD) trials ever conducted, will be presented for the first time at the Annual Congress of the European Respiratory Society (ERS), in Barcelona, Spain, September 7-11, 2013.
The highly anticipated three year TIOSPIR™ trial assessed the relative safety and efficacy of the two marketed SPIRIVA® formulations – SPIRIVA® Respimat® Soft Mist™ Inhaler 2.5 µg (once a day, 2 puffs§) versus SPIRIVA HandiHaler® 18 μg. TIOSPIR™ included more than 17,000 COPD patients** from 50 countries and, with broad inclusion criteria, featured a real-world study population that closely reflected the types of COPD patients physicians see every day. Boehringer Ingelheim is pleased to be sharing these results with the scientific community at the ERS Annual Congress, the largest respiratory meeting in the world.
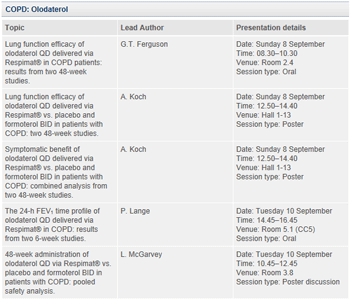
In addition, Boehringer Ingelheim will present data from the olodaterol Respimat® monotherapy Phase III clinical trial programme, which involves more than 3,000 patients with moderate to very severe COPD (GOLD 2-4). Results will be presented on lung function (FEV1) efficacy, and the symptomatic benefit of olodaterol Respimat®, as well as its safety. Olodaterol Respimat® has been designed to provide bronchodilatory benefits to COPD patients who need an alternative treatment option.
Asthma
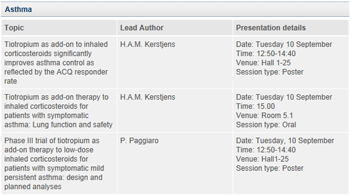
Further highlights from abstracts presented at ERS include Phase III data from the MezzoTinA-asthma® trials – designed to evaluate the efficacy and safety of tiotropium Respimat® in asthma patients who remain symptomatic despite treatment with moderate-dose maintenance ICS therapy (400-800 μg budesonide equivalent). The results include lung function, asthma control and safety data, adding to evidence from the on-going Phase III trial programme that has shown efficacy and safety of tiotropium Respimat® in patients with asthma who continue to experience symptoms despite treatment with at least ICS/LABA††. 3
Treatment of respiratory diseases has been a major area of focus for Boehringer Ingelheim for more than 90 years and significant resources are dedicated to research in this field. In addition to novel treatments for COPD, Boehringer Ingelheim has also branched out into developing treatment options for other airway diseases, including asthma, lung cancer, idiopathic pulmonary fibrosis (IPF) and other respiratory diseases.
Lung cancer
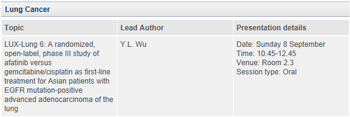
The latest clinical research data for afatinib, a targeted cancer therapy from Boehringer Ingelheim’s own research and development pipeline, will be presented at ERS. The LUX-Lung 6 trial investigates afatinib in late-stage lung cancer patients who have a specific mutation of the receptor for the epidermal growth factor (EGFR). LUX-Lung 6 is the second Phase III trial to show superiority of afatinib over standard chemotherapy in patients with EGFR mutation-positive non-small cell lung cancer (NSCLC).1,2
More people worldwide die from lung cancer than from any other form of cancer. It accounts for 1.6 million new cancer cases annually. The prevalence of tumours harbouring EGFR mutations is between 10-15% in Caucasian and 40% in Asian NSCLC patients. 4
Afatinib is approved in the US under the brand name GILOTRIF™ and in Taiwan under the brand name GIOTRIF® for use in patients with distinct types of non-small cell lung cancer. The Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency has issued a positive opinion for afatinib and regulatory reviews by health authorities in other countries are ongoing.
IPF
Boehringer Ingelheim is conducting Phase III clinical research in IPF, a rare and deadly disease affecting between 14 and 43 people per 100,000 worldwide.5 Nintedanib‡‡ is an investigational small molecule tyrosine kinase inhibitor (TKI) currently being studied in two pivotal Phase III clinical trials (INPULSIS™-1 and 2) for the treatment of IPF. Nintedanib has been awarded orphan-drug status in the EU, Japan and US. In June 2013, nintedanib was granted Fast Track designation by the US Food and Drug Administration (FDA). The FDA’s Fast Track program is designed to expedite the development and review of drugs to treat serious life-threatening diseases and to meet an unmet medical need.6
An educational website for health care professionals to support early diagnosis of IPF is available online following this link: www.soundsofipf.com
The ERS annual congress is the largest respiratory meeting in the world and is an important platform for professional exchange. Boehringer Ingelheim’s abstracts in respiratory research can be accessed through the ERS website after 31 August, www.erscongress2013.org.
Boehringer Ingelheim will present a total of 21 abstracts at ERS and host three media briefings to contextualise important data results throughout the congress, underscoring the company’s focus on finding answers to the challenges faced by patients living with lung disease.
Key sessions are highlighted below.
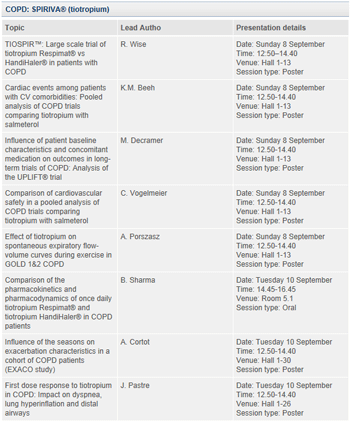




About SPIRIVA® (tiotropium bromide) in COPD
SPIRIVA®, a long-acting anticholinergic medication, is the first inhaled maintenance treatment to provide significant and sustained improvements in lung function with once-daily dosing. SPIRIVA® positively impacts the clinical course of COPD, helping to change the way patients live with their disease.7 It is the most prescribed maintenance therapy for COPD worldwide.8 SPIRIVA® helps COPD patients breathe more easily by opening narrowed airways and helping to keep them open for 24 hours. SPIRIVA® works through targeting of a dominant reversible mechanism of COPD – cholinergic bronchoconstriction.
SPIRIVA® is delivered via HandiHaler®, a breath-actuated, single-dose dry powder inhaler, or by SPIRIVA® Respimat® Soft Mist Inhaler™ propellant-free, new generation inhaler that combines innovative technology with the proven efficacy of SPIRIVA®.
About olodaterol
Olodaterol is an investigational, highly selective, long-acting beta2-agonist (LABA) currently being studied as a once-daily maintenance bronchodilator treatment of airflow obstruction in patients with chronic obstructive pulmonary disease (COPD), including chronic bronchitis and/or emphysema.
Boehringer Ingelheim is developing olodaterol as a combination partner for tiotropium to provide additional bronchodilation in the maintenance treatment of patients with COPD. Combining a long-acting anticholinergic (LAMA), such as SPIRIVA®, and a LABA has the potential to deliver increased bronchodilatory benefits in terms of COPD symptoms and consistency of treatment response versus monotherapy.
About tiotropium in asthma
Phase III clinical trials of tiotropium in asthma are underway to investigate its efficacy and safety in asthma patients who remain symptomatic despite ICS therapy (with long-acting beta2-agonist (LABA) or ICS alone).
The tiotropium in asthma clinical development programme is being jointly developed by Boehringer Ingelheim and Pfizer with Boehringer Ingelheim managing the operations for all clinical development activities. The existing alliance contract between Boehringer Ingelheim and Pfizer allows for commercialisation by both companies.
About afatinib in lung cancer
Afatinib is an irreversible ErbB Family Blocker which irreversibly blocks EGFR (ErbB1) as well as the other relevant members of the ErbB Family that are known to play a critical role in the growth and spread of the most pervasive cancers and cancers associated with high mortality, including lung cancer. The LUX-Lung clinical trial programme is investigating afatinib in a number of patient populations with advanced non-small cell lung cancer (NSCLC). Two pivotal Phase III studies have recently been presented from the LUX-Lung clinical programme. LUX-Lung 6 (n=364) and LUX-Lung 3 (n=345) represent the largest, most robust and consistent clinical registration trial programme in EGFR mutation positive NSCLC to date.
About nintedanib in IPF
Idiopathic pulmonary fibrosis (IPF) is a deadly orphan disease, with a prevalence varying between 14 and 43 people per 100,000 worldwide.9 Around 50% of patients die within 2-3 years after diagnosis.10 The average time to diagnosis is 1-2 years after the onset of symptoms,11,12 yet early referral to a specialist centre is associated with improved patient survival.13
Nintedanib is an investigational small molecule tyrosine kinase inhibitor (TKI) which targets growth factor receptors that have been shown to potentially be involved in pulmonary fibrosis.14 Nintedanib has orphan-drug status in the EU, Japan and US. In June 2013, nintedanib was given Fast Track designation by the US Food and Drug Administration.
The pivotal Phase III sister trials INPULSIS™-1 and INPULSIS™-2, investigating the efficacy and safety of nintedanib in IPF, are ongoing. Results are expected to become available in 2014.
References
- Wu YL, Zhou C, Hu CP, et al. LUX-Lung 6: A randomized, open-label, Phase III study of afatinib (A) vs. gemcitabine/cisplatin (GC) as first-line treatment for Asian patients (pts.) with EGFR mutation-positive (EGFR M+) advanced adenocarcinoma of the lung.(Abstract #8016) at American Society of Clinical Oncology, Chicago, June 2, 2013.
- Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008;26:3543–51.
- Kerstjens HAM, Engel M, Dahl R, et al. Tiotropium in asthma poorly controlled with standard combination therapy. N Engl J Med 2012; 367 (13): 1198-1207
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893-917.
- Raghu G, Weycker D, Edelsberg J, et al. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2006;174:810-6
- FDA. Fast Track, Breakthrough Therapy, Accelerated Approval and Priority Review. Accessed at: http://www.fda.gov/forconsumers/byaudience/forpatientadvocates/speedingaccesstoimportantnewtherapies/ucm128291.htm. Accessed August 2013.
- Vincken W, van Noord JA, Greefhorst APM, et al. Improved health outcomes in patients with COPD during 1 year’s treatment with tiotropium. Eur Respir J 2002;19:209-216
- IMS Health, IMS MIDAS™, Q2 2012.
- Raghu G, Weycker D, Edelsberg J, et al. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2006;174:810-6.
- Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788-824
- Collard HR, Tino G, Noble P, et al. Patient experiences with pulmonary fibrosis. Respir Med 2007;101:1350–4
- Ley B, Collard HR, King TE, Jr. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2011;183:431–40
- Lamas D, Kawut S, Bagiella E, et al. Delayed Access and Survival in Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med 2011; 184:842–847
- Selman M, King TE, Pardo A, et al. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med 2001;134(2):136-51.
* Olodaterol (Striverdi®) Respimat® is approved for use in COPD in Canada and Russia; approval and regulatory reviews by health authorities in the US, Europe and other countries are pending
† Tiotropium delivered by Respimat® is not approved for the treatment of asthma
‡ In the USA and in Taiwan, afatinib is approved for use in patients with distinct types of non-small cell lung cancer. Afatinib is under regulatory review by EMA and by health authorities in other countries
§ This is the marketed dose referred to in the NEJM TIOSPIR™ publication as tiotropium Respimat® 5 µg
**A third treatment arm included an investigational dose of SPIRIVA® Respimat®, 1.25 µg (once a day, 2 puffs), referred to in the NEJM TIOSPIR™ publication as tiotropium Respimat® 2.5 µg
††Long-acting beta2-agonists
‡‡Please note: nintedanib is not licensed for the treatment of idiopathic pulmonary fibrosis and is not yet approved




