Merck announces presentation of interim data from study evaluating Lambrolizumab
Posted: 2 June 2013 | | No comments yet
Merck expands Lambrolizumab clinical development program…


Merck (NYSE: MRK), known as MSD outside the United States and Canada, today announced the presentation of preliminary results from an ongoing Phase IB expansion study evaluating the safety and efficacy of lambrolizumab (MK-3475), Merck’s investigational antibody therapy targeting PD-1, in patients with advanced (inoperable and metastatic) melanoma. The data were presented by Antoni Ribas, M.D., Ph.D., professor, Hematology/Oncology and Surgery, and director of the Tumor Immunology Program at the Jonsson Comprehensive Cancer Center, University of California, Los Angeles, during an oral session at the American Society of Clinical Oncology (ASCO) 2013 Annual Meeting in Chicago (Abstract# 9009). The study was also published online in the New England Journal of Medicine.
“We are encouraged by the results observed to date, including the rate and duration of responses, in patients with advanced melanoma,” said Roger M. Perlmutter, M.D. Ph.D., president, Merck Research Laboratories. “Based on these data and additional findings from our ongoing studies, Merck plans to initiate late-stage clinical trials of lambrolizumab in advanced melanoma, and non-small cell lung cancer in the third quarter of 2013.”
Data from the ongoing multi-center, single-arm open-label Phase IB trial were presented from 135 patients with advanced melanoma enrolled in the study and who were administered an initial dose of lambrolizumab between Dec. 1, 2011, and Sept. 6, 2012. Patients received one of three dosing regimens of lambrolizumab (10mg/kg every two weeks [10mg/kg Q2W]; 10mg/kg every three weeks [10mg/kg Q3W] or 2mg/kg every three weeks [2mg/kg Q3W]) until disease progression or unacceptable toxicity. Tumor response was assessed every 12 weeks by independent, central, blinded radiographic review per RECIST 1.1 (Response Evaluation Criteria in Solid Tumors) criteria as well as investigator-assessed, immune-related response criteria. Earlier interim data from this study were presented at the 9th International Congress of the Society for Melanoma Research (SMR) in Hollywood, Calif., in November 2012.
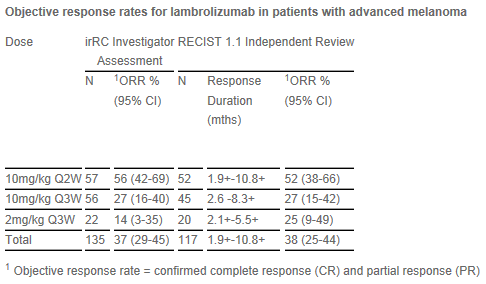

The interim overall confirmed response rate for lambrolizumab treatment was 38 percent (range 25–44 percent), as measured by RECIST 1.1 independent review across all dosing regimens evaluated. The highest overall response rate observed was 52 percent, which occurred in the 10 mg/kg Q2W dosing regimen. Ten percent of patients in this dose group were complete responders. The duration of confirmed responses, as measured after the first 12 week evaluation, ranged from greater than 28 days to up to more than 8 months at the time of the analysis, with 80 percent of responding patients continuing on treatment. The median duration of response has not been reached with a median follow-up time of 11 months. The most common treatment-related adverse events observed for those patients evaluable for adverse events, as of December 2012, were mostly grade 1/2 and included fatigue (30%), rash (21%), pruritus (21%) and diarrhea (20%). Six (4.4%) treatment-related cases of pneumonitis were reported (all grade 1/2). A total of 17 (13%) grade 3/4 treatment-related adverse events were reported, the most common of these were fatigue (1.5%), rash (2%) and elevated AST levels (1.5%). The majority of reported grade 3/4 events occurred in patients administered 10mg/kg Q2W.
The response rates for ipilimumab-pretreated and ipilimumab-naïve patients treated with lambrolizumab were similar.
“We continue to make significant progress in our clinical development program for lambrolizumab in multiple indications,” said Gary Gilliland, M.D., Ph.D., senior vice president and oncology franchise head, Merck Research Laboratories. “We are very grateful to the patients, investigators, regulators and the broader oncology community for their ongoing collaboration and support in advancing this program.”
Lambrolizumab Clinical Development Program
Merck is currently conducting four clinical trials evaluating lambrolizumab in multiple cancer types that involve more than 600 patients. The company recently initiated a global, randomized, Phase II clinical trial of lambrolizumab versus standard chemotherapy for patients with advanced melanoma whose disease has progressed after prior therapy (ref: NCT01704287) and a Phase I trial of lambrolizumab for the treatment of triple negative breast, metastatic bladder cancer and head & neck cancer (ref: NCT01848834). Further late-stage studies including a Phase III study evaluating lambrolizumab in ipilimumab-naïve patients with advanced melanoma and a Phase II/III trial in non-small cell lung cancer (NSCLC) are scheduled to commence in the third quarter of 2013. Additional trials in several hematological cancers and evaluation of lambrolizumab-containing combinations are scheduled to start this year. Merck announced that lambrolizumab had received Breakthrough Therapy designation from the U.S. Food and Drug Administration for advanced melanoma in April 2013.
Merck Oncology Briefing Webcast
Merck will hold a briefing on June 2 at 1:00 p.m. CDT to present an update of ongoing progress in the development of its oncology candidates MK-1775 and vintafolide, as well as provide details regarding its clinical development plans for lambrolizumab. Investors and journalists may access a live audio webcast of the event on Merck’s website at http://www.merck.com/investors/events-and-presentations/home.html. Software needed to listen to the webcast is available on the corporate website and should be downloaded prior to the beginning of the webcast. A replay of the webcast will be available at approximately 8:00 a.m. EDT on June 3.
About Lambrolizumab
Lambrolizumab is an investigational antibody therapy designed to disrupt the action of the immune checkpoint protein PD-1 and therefore inhibit the ability of some cancers to evade the body’s immune system. Lambrolizumab is being studied in multiple cancer types including melanoma and non-small cell lung cancer. For further details, please visit http://clinicaltrials.gov.
About Breakthrough Therapy Designation
The designation of an investigational drug as a Breakthrough Therapy is intended to expedite the development and review of a candidate that is planned for use, alone or in combination, to treat a serious or life-threatening disease or condition when preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints. The Food and Drug Administration Safety and Innovation Act (FDASIA) includes a provision that allows sponsors to request that an investigational drug be designated as a Breakthrough Therapy. The implications of Breakthrough Therapy Designation cannot be determined at this time.
About PD-1
Researchers have shown that several tumor types are able to hide in plain sight by establishing a “molecular camouflage” that deceives the body’s immune system into thinking they are normal and therefore allow them to grow unchecked. The interaction between the immune checkpoint receptor PD-1 and its ligands represents a potentially important tumor-specific immunomodulatory mechanism. By utilizing the PD-1 pathway, a tumor cell can prevent the activation of T-cells and therefore may block a key step that triggers the immune system.
About Advanced Melanoma
Advanced melanoma accounts for more than 80 percent of skin cancer-related deaths and one to two percent of all cancer deaths in the United States. According to the American Cancer Society, an estimated 9,180 people in the U.S. died from advanced melanoma in 2012.




