MHRA authorises novel pre-filled pen injector
Posted: 21 September 2023 | Catherine Eckford (European Pharmaceutical Review) | No comments yet
Adtralza®(tralokinumab) has been granted a UK marketing authorisation by the Medicines and Healthcare Products Regulatory Agency (MHRA) to treat atopic dermatitis, halving the number of injections required.
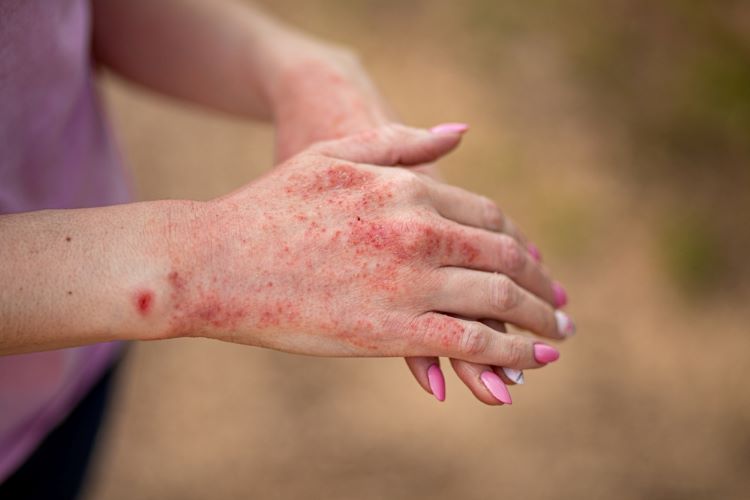

The Medicines and Healthcare Products Regulatory Agency (MHRA) has granted marketing authorisation for 300mg of Adtralza® (tralokinumab) in 2mL solution for injection in a new pre-filled pen.
Approval by the MHRA follows the European Commission (EU) decision made on the 1 September 2023.
Tralokinumab is indicated for the treatment of moderate-to-severe atopic dermatitis in adults and adolescent 12 years and over who are candidates for systemic therapy.
The monoclonal antibody biologic treatment has been developed to bind to and inhibit the interleukin (IL)-13 cytokine. It inhibits interaction with the IL-13 receptor α1 and α2 subunits (IL-13Rα1 and IL-13Rα2). IL-13 has a role in the immune and inflammatory processes underlying atopic dermatitis signs and symptoms.
Higher efficiency tralokinumab drug delivery
Tralokinumab is at present, available in a 150mg in 1 mL pre-filled syringe, requiring four initial, and two maintenance doses with the 150mg in 1mL pre-filled syringe. Instead, the new 300mg in 2 mL, followed by a single 300mg maintenance dose given every other week. This results in administration requiring half the number of injections currently required.
New features of the new pre-filled pen consist of a hidden needle and a press-down auto-injection with visual and audible feedback mechanisms to aid patients with drug delivery.
“We are pleased that this new simplified method of administration for tralokinumab will soon be available for appropriate atopic dermatitis patients in the UK as we strive to advance the standard of care and support for people living with skin conditions,” shared Leanne Walsh, Vice President and General Manager of UK and Ireland at Denmark-based pharmaceutical company LEO Pharma.
The new administration method will be available in the UK for tralokinumab patients from early 2024.
Atopic dermatitis
This chronic, inflammatory skin disease is characterised by symptoms such as intense itch and eczematous lesions. Type 2 cytokines, including IL-13, play an important role in atopic dermatitis pathophysiology.
UK and Europe approve Adtralza for treating atopic dermatitis
Related topics
Big Pharma, Biopharmaceuticals, Drug Delivery Systems, Drug Safety, Industry Insight, Regulation & Legislation, Research & Development (R&D), Therapeutics
Related organisations
LEO Pharma, Medicine and Healthcare products Regulatory Agency (MHRA)









