Semaglutide demonstrates cardiovascular benefit
Posted: 8 August 2023 | Catherine Eckford (European Pharmaceutical Review) | No comments yet
In Novo Nordisk’s landmark trial for cardiovascular outcomes, semaglutide 2.4mg has potential to change how obesity is regarded and treated, headline data suggests.
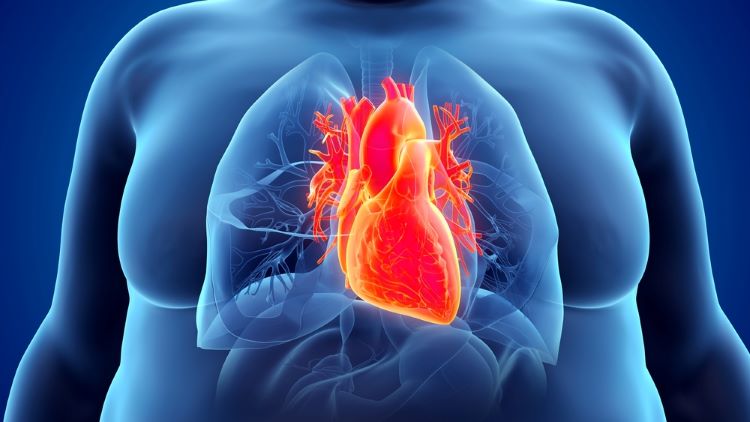

Headline results from Novo Nordisk’s “landmark” SELECT trial in cardiovascular outcomes has demonstrated a 20 percent reduction in major adverse cardiovascular events (MACEs) for semaglutide 2.4mg (Wegovy®) compared to a placebo.
“People living with obesity have an increased risk of cardiovascular disease but to date, there are no approved weight management medications proven to deliver effective weight management while also reducing the risk of heart attack, stroke or cardiovascular death,” commented Martin Holst Lange, Executive Vice President for Development at Novo Nordisk.
The SELECT clinical trial enrolled 17,604 overweight or obese adults aged ≥45 years. The subcutaneous once-weekly treatment was evaluated as an adjunct to standard of care (SOC) in these patients for up to five years.
Participants who were enrolled in the clinical trial had a BMI ≥27 kg/m2 and cardiovascular disease (CVD) with no previous history of diabetes.
“SELECT is a landmark trial and has demonstrated that semaglutide 2.4mg has the potential to change how obesity is regarded and treated,” stated Lange.
About Wegovy semaglutide 2.4mg
Current indications and regulatory approvals
The GLP-1 receptor agonist is indicated as an adjunct treatment to a reduced calorie diet and increased physical activity for chronic weight management in obese adults with a BMI of 30 kg/m2 or higher, overweight adults with a BMI of 27 kg/m2 or greater, in the presence of at least one weight-related comorbid condition. It is also indicated to treat obese paediatric patients over 12 years old with an initial BMI at the 95th percentile or greater for age and gender.
Currently, Wegovy is launched in Denmark, Norway, Germany as well as the US. Novo Nordisk has stated it expects to file for a regulatory label indication expansion for the treatment in the US and the EU in 2023.
Detailed results from the SELECT clinical trial will be presented at a scientific conference later in 2023.
Related topics
Big Pharma, Biopharmaceuticals, Clinical Development, Clinical Trials, Data Analysis, Drug Development, Industry Insight, Research & Development (R&D), Therapeutics