US begins monkeypox vaccine and therapeutic trials
Posted: 12 September 2022 | Hannah Balfour (European Pharmaceutical Review) | No comments yet
US National Institutes of Health initiates trials to evaluate antiviral tecovirimat (TPOXX) and possibility of delivering Jynneos vaccine intradermally.
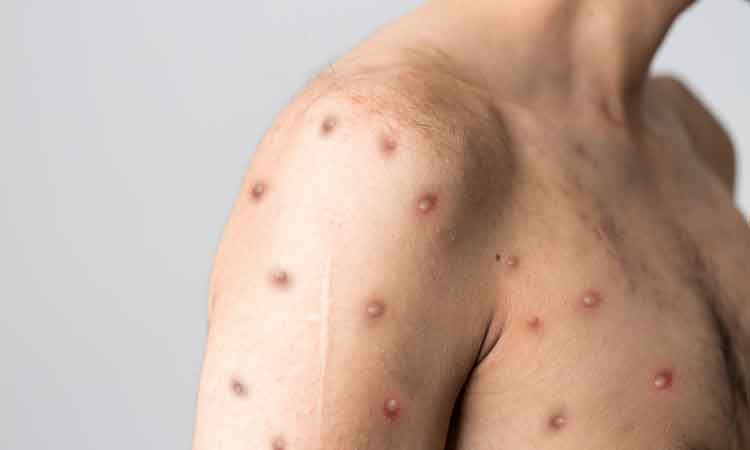

The US National Institutes of Health (NIH) announced the initiation of two trials in aid of the efforts to curb monkeypox. The first aims to explore whether intradermal delivery of the Jynneos monkeypox vaccine is efficacious, and could thereby be used to expand the limited vaccine supply; while the second aims to establish the efficacy of SIGA’s antiviral tecovirimat (TPOXX).
A global outbreak of human monkeypox virus, first identified in May 2022, prompted public health emergency declarations from both the World Health Organization (WHO) and the US Department of Health and Human Services this past summer. The global outbreak is primarily affecting men who have sex with men and the Centers for Disease Control and Prevention (CDC) has reported 20,733 cases of monkeypox in the US since the start of the outbreak.
The virus usually causes painful skin lesions and flu-like symptoms. Serious complications can include dehydration, bacterial infections, pneumonia, brain inflammation, sepsis, eye infections and death. Historically, the virus is known to be transmitted from person to person through direct contact with skin lesions, body fluids and respiratory droplets, as well as by indirect contact with items such as contaminated clothing or bedding. Preliminary analyses indicate that sexual transmission may be playing a role in the current outbreak.
Intradermal vaccine delivery
A clinical trial evaluating alternative strategies for administering Bavarian Nordic’s Jynneos monkeypox vaccine to increase the number of available doses has begun enrolling adult volunteers (aged 18 to 50 years). The National Institute of Allergy and Infectious Diseases (NIAID) trial (NCT05512949) will enrol more than 200 adults across eight US research sites.
Jynneos is approved by the US Food and Drug Administration for administration by two subcutaneous injections 28 days apart for the prevention of smallpox and monkeypox disease in adults 18 years of age and older determined to be at high risk for smallpox or monkeypox infection.
FDA recently authorised intradermal administration of the vaccine for adults, this alternative dosing regimen uses one-fifth of the standard dose used for subcutaneous administration, expanding the number of people who can be vaccinated with the currently limited supply. The approval was based on data from a 2015 NIH publication.
“To halt the global outbreak of monkeypox and to help protect those at risk of infection, we need to ensure we have an adequate supply of monkeypox vaccine,” commented NIAID Director Dr Anthony Fauci. “NIAID’s trial of Jynneos will provide important information on the immunogenicity, safety and tolerability of alternative dosing approaches that would expand the current supply of vaccine.”
Adults who have not been vaccinated against smallpox or monkeypox previously are eligible to enrol in the NIAID trial. All trial participants will receive the Jynneos vaccine in some form and will be randomly assigned to one of three study arms:
- standard, licensed regimen – 1×108 infectious virus particles administered subcutaneously
- the regimen recently authorised by the FDA – 2×107 infectious virus particles (one-fifth of the standard regimen) administered intradermally
- one-tenth (1×107 infectious virus particles) of the standard regimen of Jynneos administered intradermally.
Investigators will assess whether the peak immune responses induced in recipients receiving the vaccine intradermally are at least as good as those induced by the licensed subcutaneous regimen and will compare the relative safety and tolerability of the different regimens.
Exploring antiviral efficacy
A Phase III trial of TPOXX has also begun – adults and children of any age with monkeypox are eligible to enrol in the study (A5418).
Tecovirimat, manufactured by SIGA Technologies Inc, is approved by the FDA for the treatment of smallpox – a related poxvirus. The drug prevents the virus from spreading in the body by preventing virus particles from exiting human cells by targeting a protein found on both the variola virus, which causes smallpox, and the monkeypox virus. Clinicians currently can access tecovirimat for US patients with monkeypox through an expanded access process.
Commenting on the launch of the trial, Dr Fauci stated: “We currently lack efficacy data that would help us understand how well this drug may mitigate painful monkeypox symptoms and prevent serious outcomes. This clinical trial was designed to answer those important questions.”
Adults with severe monkeypox virus infection or those at high risk for severe disease, including individuals with underlying immune deficiency, a history of or active inflammatory skin conditions; pregnant people; and children all will be enrolled in an open-label arm in which all participants receive tecovirimat. Another set of 530 adult participants will be randomly assigned in a 2:1 ratio to receive tecovirimat or placebo pills. Tecovirimat capsules are taken by mouth for 14 days, and the dose is based on the participant’s weight. This part of the trial is double-blind, meaning neither participants nor investigators will know who is receiving placebo or tecovirimat.
Investigators will gather data to determine if participants receiving tecovirimat heal more quickly (all lesions scabbed over or flaked off) compared with those taking placebo. They also will examine tecovirimat’s impact on pain scores, rates of progression to severe disease, clearance of monkeypox virus from various samples, and its safety, among other data. This study also will provide critical data on the optimal dosing and safety of tecovirimat in children and people who are pregnant.
Data on the safety and efficacy of tecovirimat will be submitted to the FDA.
The NIAID also revealed that it is collaborating with the National Institute for Biomedical Research (INRB) in the Democratic Republic of the Congo to initiate a separate clinical trial of tecovirimat in adults and children with monkeypox in that country.
Related topics
Biologics, Clinical Development, Clinical Trials, Drug Development, Drug Safety, Drug Targets, Research & Development (R&D), Therapeutics, Vaccines, Viruses
Related organisations
Bavarian Nordic, SIGA Technologies Inc., US National Institute of Allergy and Infectious Disease (NIAID), US National Institutes of Health (NIH)









