Dupixent® reduces itch by 37 percent in prurigo nodularis patients
Posted: 22 October 2021 | Anna Begley (European Pharmaceutical Review) | No comments yet
Dupixent® is the first biologic to significantly reduce itch and skin lesions in Phase III trial for prurigo nodularis.
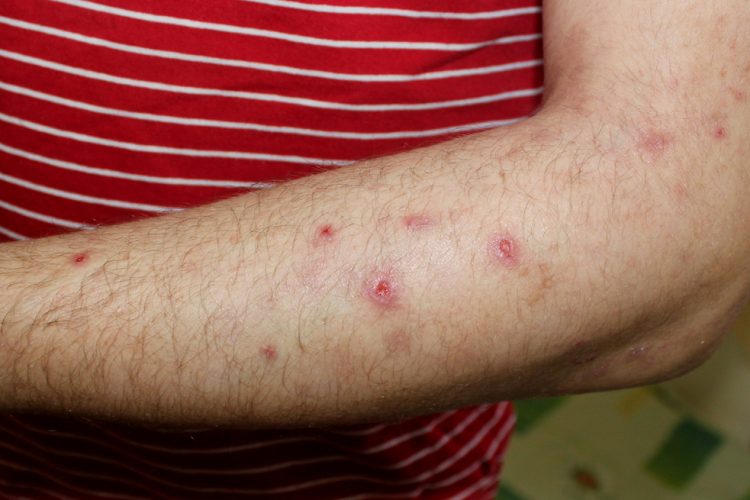

Sanofi’s Dupixent® has been shown to significantly reduce itch and skin lesions compared to placebo in (dupilumab) in adults with uncontrolled prurigo nodularis, a chronic type 2 inflammatory skin disease that causes extreme itch and skin lesions, in a pivotal Phase III trial.
PRIME2 is a randomised, Phase III, double-blind, placebo-controlled trial that evaluated the efficacy and safety of Dupixent in 160 adults with prurigo nodularis inadequately controlled with topical prescription therapies or with whom those therapies are not advisable.
During the 24-week treatment period, prurigo nodularis patients received Dupixent or placebo every two weeks with or without topical treatments (low- or medium-dose topical corticosteroids or topical calcineurin inhibitors were continued if patients were using these treatments at randomisation.
Topline results comparing Dupixent (n=78) to placebo (n=82) showed:
- 37 percent of Dupixent patients experienced a clinically meaningful reduction in itch from baseline compared to 22 percent of placebo patients at week 12, the primary endpoint
- At week 24, nearly three times as many Dupixent patients experienced a clinically meaningful reduction in itch from baseline: 58 percent of Dupixent patients compared to 20 percent of placebo patients
- At 24 weeks, Dupixent patients were nearly three times as likely to achieve clear or almost clear skin: 45 percent of Dupixent patients compared to 16 percent of placebo patients
- Dupixent patients experienced significantly greater improvements in measures of health-related quality of life, skin pain and symptoms of anxiety and depression.
The safety results of the trial were generally consistent with the known safety profile of Dupixent in its approved indications. The most common adverse events were conjunctivitis, herpes viral infections and skin infections. Additionally, three percent of Dupixent patients and 30 percent of placebo patients discontinued prior to week 24.
Dupixent is a fully human monoclonal antibody that inhibits the signalling of the interleukin-4 (IL-4) and interleukin-13 (IL-13) pathways and is not an immunosuppressant. IL-4 and IL-13 are key and central drivers of the type 2 inflammation that plays a major role in atopic dermatitis, asthma, and chronic rhinosinusitis with nasal polyposis (CRSwNP).
“We are encouraged that patients in this trial experienced a significant reduction in itch and skin lesions, especially given that prior to enrolment nearly all patients had severe itch and nearly 40 percent had 100 or more nodules covering their body,” commented Dr John Reed at Sanofi. “These data are an important step forward in furthering our knowledge of the role that targeting IL-4 and IL-13 can play in the treatment of skin diseases that cause extreme itch.”
Related topics
Big Pharma, Biopharmaceuticals, Clinical Trials, Data Analysis, Drug Safety, Research & Development (R&D), Therapeutics