UK and Europe approve Adtralza for treating atopic dermatitis
Posted: 23 June 2021 | Hannah Balfour (European Pharmaceutical Review) | No comments yet
EU and UK regulators have approved Adtralza (tralokinumab), a monoclonal antibody, for treating moderate-to-severe atopic dermatitis in adult patients.
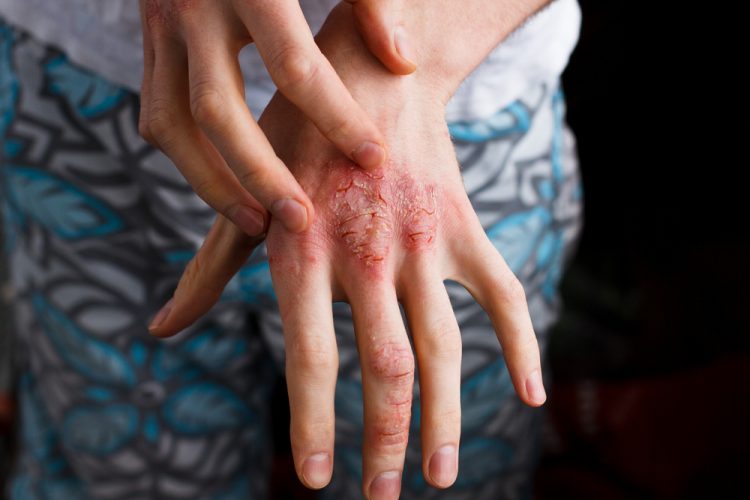

The UK’s Medicines and Healthcare products Regulatory Agency (MHRA) and the European Commission (EC) have both approved Adtralza (tralokinumab) for the treatment of moderate-to-severe atopic dermatitis in adult patients who are candidates for systemic therapy. The approvals make tralokinumab the first and only approved biologic that specifically targets the interleukin 13 (IL-13) cytokine, a key driver of atopic dermatitis signs and symptoms.
Atopic dermatitis is a chronic, inflammatory, skin disease characterised by intense itch and eczematous lesions. The chronic inflammation is caused by skin barrier dysfunction and immune dysregulation. Type 2 cytokines, including IL-13, play a central role in the key aspects in its pathophysiology.
Tralokinumab is the first high affinity, human monoclonal antibody developed to specifically bind to and inhibit the IL-13 cytokine in adult patients with uncontrolled moderate-to-severe atopic dermatitis. Tralokinumab will be available in a 150mg/mL prefilled syringe for subcutaneous injection with an initial dose of 600mg followed by 300mg every other week. Tralokinumab can be used with or without topical corticosteroids (TCS).
The approval is based primarily on safety and efficacy results from the ECZTRA 1, 2 and ECZTRA 3 Phase III trials, which included more than 1,900 adult patients with moderate-to-severe atopic dermatitis. Safety data was evaluated from a pool of five randomised, double-blind, placebo-controlled trials, including ECZTRA 1, 2 and ECZTRA 3, a dose ranging trial and a vaccine response trial.
“Atopic dermatitis can be an intensely itchy, challenging and unpredictable skin condition for some. As clinicians, we always want more options for patients and the approval of tralokinumab means that clinicians across the UK and Ireland now have an important new treatment option for patients with moderate-to-severe atopic dermatitis in adult patients,” commented Professor Anthony Bewley, Consultant Dermatologist at Barts Health NHS Trust.
LEO Pharma is working closely with key stakeholders to support access to tralokinumab for eligible patients.
Additional regulatory filings are underway with the US Food and Drug Administration (FDA) and other health authorities worldwide.
Related topics
Antibodies, Biologics, Drug Safety, Drug Targets, Regulation & Legislation, Therapeutics
Related organisations
The European Commission (EC), UK Medicines and Healthcare products Regulatory Agency (MHRA)









