Final Phase IIb interferon-free hepatitis C data from Boehringer Ingelheim to be presented at AASLD
Posted: 4 October 2012 | | No comments yet
New data from HCVersoTM have been accepted for presentation…


New data from Boehringer Ingelheim’s hepatitis C virus (HCV) clinical development programme, HCVersoTM, have been accepted for presentation at the 63rd Annual Meeting of the American Association for the Study of Liver Diseases (AASLD), taking place 9 – 13 November in Boston, MA.
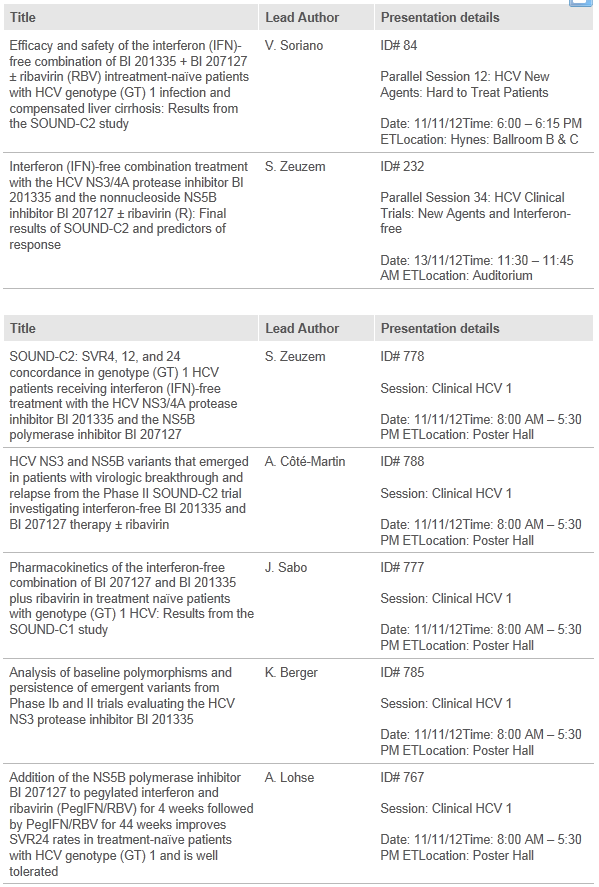
Seven abstracts will be presented at the meeting, most notably final results from the Phase IIb interferon-free SOUND-C2 trial of faldaprevir (formerly known as BI 201335) and BI 207127 with and without ribavirin.1,2 This study includes patients with the most common and most challenging type of HCV to cure, genotype-1, including patients with complete liver cirrhosis,1 an advanced form of liver disease.
Through its robust clinical trial programme, Boehringer Ingelheim aims to find solutions to the real-life challenges faced by hepatitis C patients. Phase III trials investigating the interferon-free regimen will commence later this year.
Boehringer Ingelheim’s abstracts can be accessed through the AASLD website today, www.aasld.org.

About Hepatitis C Virus (HCV)
Hepatitis C is a viral disease caused by the hepatitis C virus (HCV) that mainly affects the liver. It is a leading cause of chronic liver disease, liver cancer and transplant. The number of individuals chronically infected with HCV globally has been estimated at 150 million, with 3–4 million new infections occurring each year.3 Only about 15-25 percent of patients clear the virus in the acute phase.3 Of the remaining chronically infected patients, 20 percent will develop cirrhosis within 20 years on average.4,5 The mortality rate after cirrhosis has developed is 2-5 percent per year.5 End-stage liver disease due to HCV infection currently represents the major cause for liver transplantation in the Western world.5
About Boehringer Ingelheim in Hepatitis C Virus (HCV)
Through pioneering science, Boehringer Ingelheim strives to spread the cure in HCV and ease the burden of the disease. Boehringer Ingelheim’s clinical research team is working with HCV experts from all over the world to extend a cure to more patients suffering from the disease, including those who are toughest to cure. Boehringer Ingelheim is investigating faldaprevir (BI 201335) and BI 207127 through HCVersoTM, our rigorous clinical trial programme that is designed to find answers to the real-life challenges that HCV patients face.
Faldaprevir (BI 201335), an investigational oral HCV NS3/4A next generation protease inhibitor has shown the potential to improve cure rates as compared to PegIFN/RBV therapy alone, has completed clinical trials through Phase IIb (SILEN-C studies). The ongoing multi-study Phase III trial programme, STARTVersoTM, that evaluates faldaprevir combined with PegIFN/RBV in treatment-naïve, treatment-experienced and HIV co-infected patients with chronic genotype-1 HCV is nearly complete.
BI 207127 is an investigational non-nucleoside NS5B RNA-dependent polymerase inhibitor that has the potential to eliminate interferon from HCV treatment when combined with faldaprevir (BI 201335) and RBV. Phase II trials of this interferon-sparing regimen have been completed and Phase III HCVersoTM trials investigating this regimen will commence later this year.
References
- Soriano V. et al. Efficacy and safety of the interferon (IFN)-free combination of BI 201335 + BI 207127 ± ribavirin (RBV) in treatment-naïve patients with HCV genotype (GT) 1 infection and compensated liver cirrhosis: Results from the SOUND-C2 study. To be presented at AASLD 2012
- Zeuzem S. et al. Interferon (IFN)-free combination treatment with the HCV NS3/4A protease inhibitor BI 201335 and the nonnucleoside NS5B inhibitor BI 207127 ± ribavirin (R): Final results of SOUND-C2 and predictors of response. To be presented at AASLD 2012
- World Health Organisation. Hepatitis C Fact Sheet. Updated July 2012 http://www.who.int/mediacentre/factsheets/fs164/en/index.html [Last accessed on 27/09/12]
- National Digestive Disease Information Clearing House, NIH. Chronic Hepatitis C Current Disease Management. NIH Publication No. 10-4230. January 2010
- Soriano, Vincent et al. New Therapies for Hepatitis C Virus Infection. Clinical Infectious Disease, February 2009




