Avelumab significantly improves advanced bladder cancer patient survival
Posted: 21 September 2020 | Hannah Balfour (European Pharmaceutical Review) | No comments yet
A Phase III trial concluded that avelumab is an effective maintenance therapy for patients with advanced or metastatic urothelial carcinoma, whose disease had not progressed after chemotherapy.
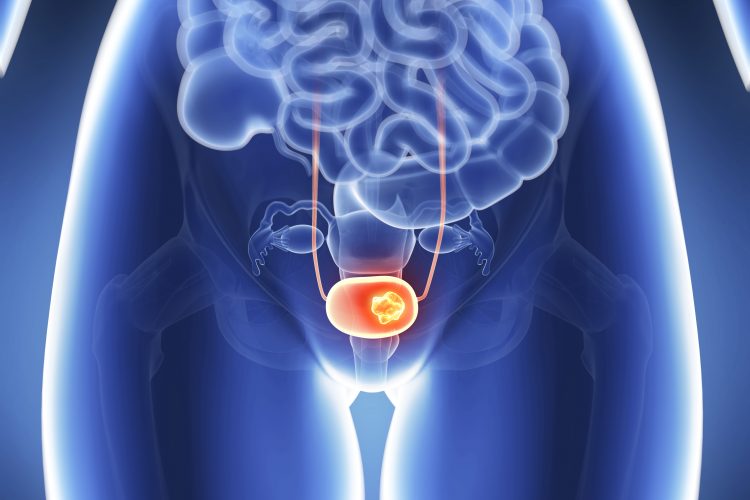

Avelumab significantly improved survival in patients with advanced or metastatic urothelial carcinoma, the most common form of bladder cancer, in a Phase III trial led by Queen Mary University of London and Barts Cancer Centre, both UK.
The results from the Phase III JAVELIN Bladder 100 trial evaluating the immunotherapy drug were published in the New England Journal of Medicine.
According to paper, the drug led to a 31 percent reduction in risk of death from bladder cancer and extended median survival in advanced bladder cancer by more than seven months. The researchers said this is the first time an immunotherapy has resulted in a survival advantage in this setting in bladder cancer and therefore this treatment could potentially benefit thousands of patients every year.
Roughly 550,000 new cases of bladder cancer are diagnosed each year worldwide. This trial focused on the group of these patients whose cancer had metastasised beyond the bladder (advanced or stage 4 disease), which is difficult to treat and results in more than 200,000 deaths each year globally.
Chemotherapy is the standard initial treatment for metastatic urothelial carcinomas, but after the chemotherapy course is finished patients must be regularly checked as the cancer tends to return quickly, be more treatment resistant and cause poor survival outcomes.
The global JAVELIN Bladder 100 trial evaluated the efficacy of the immunotherapy drug avelumab in patients with locally advanced or metastatic urothelial carcinoma, whose disease had not progressed after chemotherapy.
A total of 700 patients from over 200 sites around the world were assigned to two treatment groups after the completion of chemotherapy: one group received regular checking (standard care) alone, the other received avelumab in addition to standard care.
Treatment with avelumab resulted in a 31 percent reduction in risk of death and median overall survival of 21.4 months compared with 14.3 months in patients who did not receive the drug. Side effects were in line with expectations with immunotherapy and 11 percent of patients stopped avelumab due to treatment problems.
Study lead Thomas Powles, Professor of Genitourinary Oncology at Queen Mary University of London, and Director of Barts Cancer Centre, Barts Health NHS Trust, said: “This is the first time that an immune therapy clinical trial has shown a survival benefit for first-line therapy in metastatic bladder cancer.
“We saw a meaningful reduction in the risk of death and a significant overall survival benefit with avelumab, which underscores the potential for this immunotherapy to be practice-changing for patients. This highlights the potential benefits of a maintenance approach with avelumab in patients to prolong their lives following chemotherapy.”
The UK currently has an early access medicine scheme (EAMS) for avelumab, through which bladder cancer patients who have benefited from chemotherapy can access the drug. Following the JAVELIN trial avelumab will now be available in the UK to advanced/metastatic urothelial carcinoma patients through the EAMS scheme.
The US Food and Drug Administration (FDA) has approved avelumab for the maintenance treatment of patients with locally advanced or metastatic urothelial carcinoma that has not progressed with first-line platinum-containing chemotherapy based on the JAVELIN Bladder 100 results.
Avelumab is a checkpoint inhibitor drug, which inhibits the PD-L1 protein on the surface of tumour cells from silencing the immune response to cancer. By blocking PD-L1, the drug enables the immune system to identify and kill the cancer more easily.
Related topics
Anti-Cancer Therapeutics, Clinical Trials, Drug Safety, Drug Targets
Related organisations
Barts Cancer Centre, Queen Mary University of London, US Food and Drug Administration (FDA)