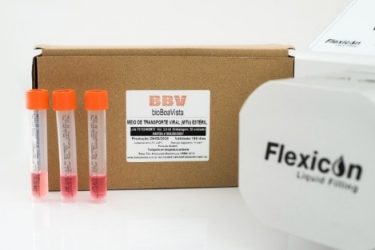
bioBoaVista manufactures customised media for COVID nasal diagnostic tests using the Flexicon PF7
Posted: 24 June 2020 | Watson-Marlow Fluid Technology Group | No comments yet
bioBoaVista showcases its viral transport medium, that can stabilise samples, for efficient nasal tests for the diagnosis of COVID-19.


bioBoaVista, a Brazilian specialist culture media manufacturer for microbiological diagnosis, has developed a viral transport medium (MTV) for more efficient nasal tests for the diagnosis of COVID-19. The novel medium stabilises samples for significantly longer; up to 48 hours at room temperature and up to 5 days between 2°C to 8°C, compared to standard saline samples that last only 12 hours.
The MTV is prepared with a cell culture medium, supplemented with proteins for viral stabilisation and the addition of antibiotics and antifungals to inhibit the growth of other microorganisms present in the nasal mucosa and the throat. This exclusive formulation prevents any interference in the stability of the virus through competition and contamination by other existing pathogens. Samples are collected using flexible plastic swabs that break inside the tube of transport, avoiding splashes and potential contamination. This process protects the quality of the sample for a longer period than traditional means, enabling more efficient transport and processing.
bioBoaVista’s diagnostic test is registered with ANVISA, the Brazilian Health Regulatory Agency. Using entirely local production means it does not depend on imports, which is of particular importance with current limited global transport.
The Company needed to efficiently fill its tubes up to the required 3ml level. bioBoaVista chose the Flexicon PF7 bench top filling machine from Watson-Marlow Fluid Technology Group (WMFTG) for its low shear, gentle pumping action. This ensures the valuable viral transport medium is transferred undamaged with high accuracy and precision.
With current batch sizes of two million units, the Company plans to increase its production to four times this and as such has scaled out capacity with additional PF7 machines. By choosing Flexicon, the Company also has the option to scale up further whilst retaining confidence in the technology. The FF30 is available to semi-automate the filling and capping process.
Precise, aseptic filling
bioBoaVista required a compact, efficient filling mechanism to fill its specialist media to the required level into the diagnostic test tubes. With precision filling from as low as 0.2ml and repeatable filling accuracies of better than 0.5 percent, the PF7 enables bioBoaVista to accurately and efficiently fill its tubes to the required level without costly overfilling.
Flexicon products are the preferred choice for the biotechnology and diagnostic industries, providing the required aseptic guarantees necessary for contamination free processes. The PF7 is the result of 30 years of industry-focused experience and comes with a five-year warranty as well as IQ/OQ documentation available on request to assist with process validation. It is designed to work with single-use fluid paths and connects to a range of balances and printers for error-free calibration to help compliance with GMP and regulatory demands.
Simple to operate
The PF7 is simple to operate, with a powerful user interface to reduce the risk of errors. Its clear and intuitive colour display and large keypad facilitate ease of use when
gowned up in cleanroom environments. This enabled bioBoaVista to fill its tubes whilst maintaining sterility to meet regulatory requirements.
Scale-out to scale-up

In order to rapidly scale-up the number of tests the Company produces, it has increased its PF7 filling capacity to seven machines that will run in parallel. This trend towards increasing capacity by scaling-out identical production lines is becoming more common with the growth of affordable, single-use technologies, a valuable development that enables rapid scale-up when time is scarce.
The Flexicon PF7 has ensured that bioBoaVista can efficiently fill its viral transport media to precise volumes whilst maintaining sterility. Its reliability and ease of use has led the Company to significantly expand its production capabilities with additional machines that will increase the number of testing kits produced by four times and facilitate essential widespread testing.
For more information, visit here.
Related topics
Biopharmaceuticals, Clinical Development, Drug Development, Good Manufacturing Practice (GMP), Industry Insight, Lab Equipment, QA/QC
Related organisations
ANVISA, bioBoaVista, Watson-Marlow Fluid Technology Group (WMFTG)









