Breakthrough Therapy Designation granted to prostate cancer drug
Posted: 7 October 2019 | Victoria Rees (European Pharmaceutical Review) | No comments yet
The FDA has given the designation to niraparib for the treatment of gene-mutated castration-resistant prostate cancer.

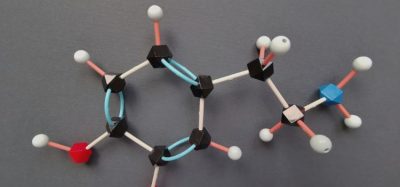
The US Food and Drug Administration (FDA) has granted Breakthrough Therapy Designation to niraparib for the treatment of patients with BRCA1/2 gene-mutated metastatic castration-resistant prostate cancer (mCRPC).
Niraparib is intended for patients who have previously received taxane chemotherapy and androgen receptor (AR)-targeted therapy. It is an orally-administered poly ADP-ribose polymerase (PARP) inhibitor.
The medication is produced by Janssen, which ran a Phase II multicentre, open-label clinical trial evaluating the efficacy and safety of niraparib. The data from this study prompted the decision by the FDA.
A Phase III trial evaluating niraparib in combination with ZYTIGA® (abiraterone acetate) and prednisone is currently ongoing.
“Niraparib is a PARP inhibitor that we believe may help address an important unmet need for patients with metastatic castration-resistant prostate cancer who have mutations in DNA-repair genes,” said Kiran Patel, MD, Vice President, Clinical Development, Solid Tumours, Janssen R&D. “We are pleased with the FDA’s Breakthrough Therapy Designation as we continue the clinical development of niraparib and we look forward to working with the agency in our continued focus and commitment to bring new advancements to patients diagnosed with prostate cancer.”
Related topics
Clinical Development, Clinical Trials, Drug Development, Regulation & Legislation, Research & Development (R&D)