Demand for containment facilities and manufacturing to grow
Posted: 17 September 2019 | Victoria Rees (European Pharmaceutical Review) | No comments yet
The market for containment facilities and containment manufacturing will increase in the future, due to a growing demand for oncology treatments.


A recent report has shown that the growing oncology therapy pipeline will have a beneficial impact on containment facilities. The research also shows that the demand for containment manufacturing will increase in the future.
…the demand for containment manufacturing will increase in the future”
The research was conducted by GlobalData, which studied a total of 1,754 small molecule active pharmaceutical ingredient (API) contract manufacturing facilities. Twenty-nine percent of facilities were found to offer containment capabilities and seven percent offered controlled substance capabilities.
AMRI, Apeloa and Patheon are highlighted by the report as being among the contract manufacturing organisations (CMOs) with the highest number of containment facilities that are able to handle substances including antibiotics, cytotoxins and steroids.
China was found to have the largest number of small molecule API manufacturing facilities, followed by India and then the US. This is despite the US having 31 percent of small molecule API facilities, compared to China’s 26 percent and 20 percent in India.
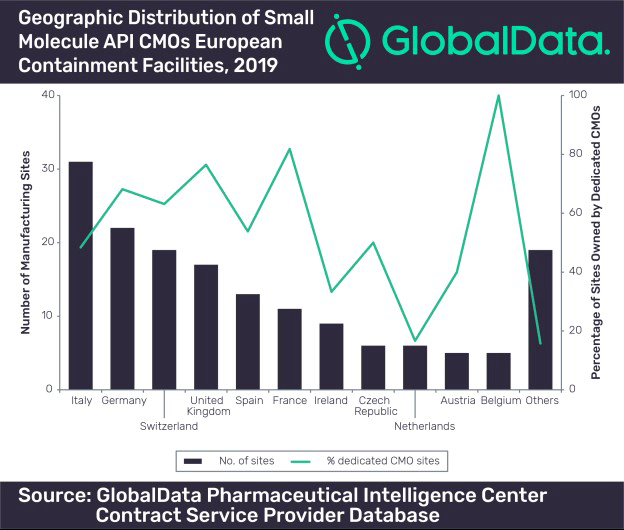

Note: Only facility counts for companies engaged in contract manufacturing are included in this figure. Dedicated CMOs only perform contract manufacturing, not both manufacturing and marketing their own products (credit: GlobalData).
Adam Bradbury, PharmSource analyst at GlobalData, said: “The emerging markets of China and India provide much of the small molecule API supply globally as well as lower-cost labour and fewer regulations than in North America… The savings are passed on to the consumer and generics can be sold cheaper in an already competitive environment.”
Bradbury continues: “Controlled substance capabilities and facility requirements can be prohibitively expensive. As such, only the largest CMOs can acquire, construct, and/or maintain a controlled substance facility. Therefore, any reaction in the US involving a reduction in their production will negatively affect the larger CMOs involved.”
Related topics
Active Pharmaceutical Ingredient (API), Contract Manufacturing, Drug Manufacturing, Drug Markets, Manufacturing









