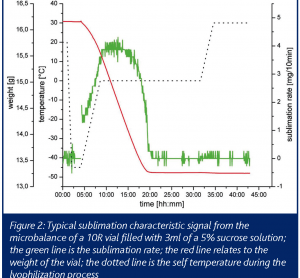
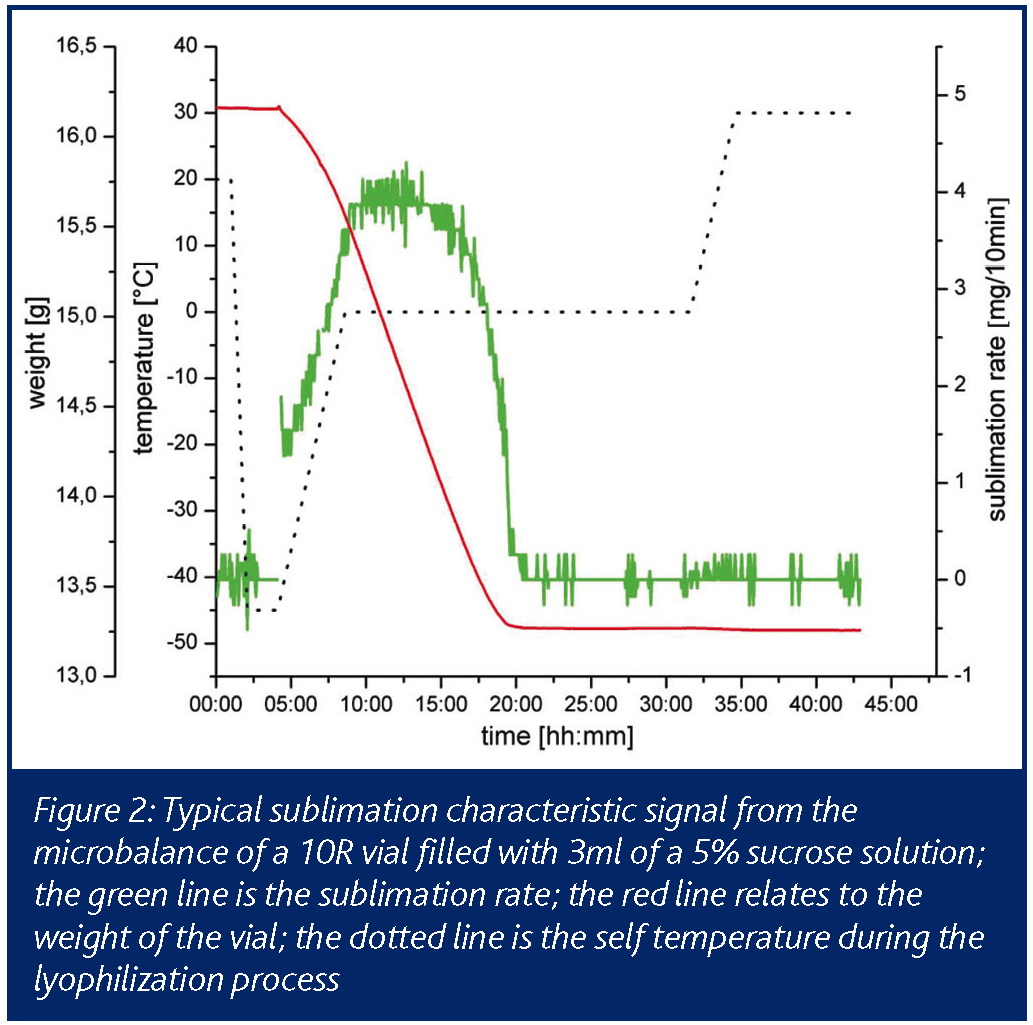
Applications in target ID and validation
11 November 2005 | By Oliver C. Steinbach, Head of Department, Technology Management, ALTANA Pharma AG
RNAi technology provides the ‘loss of function’ approach, which has been widely used in the last couple of years for analysis of gene function, and in drug discovery for identification and validation of potential drug target candidates. This technology is now widely applied for functional screens in order to identify…