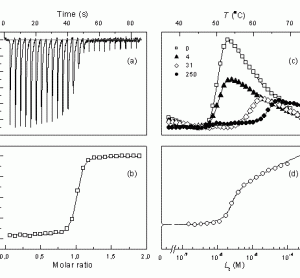
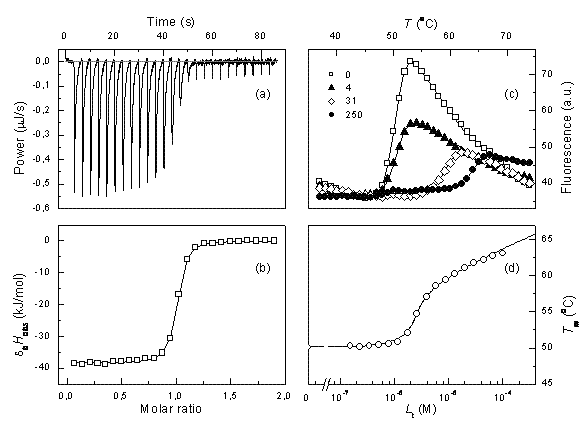
PAT & QbD Supplement (free to view)
20 June 2011 | By Magida Zeaiter - GlaxoSmithKline, Mark Morton - Phoenix Scientific Services, Joachim Ermer - Head of Quality Control Services Frankfurt Chemistry - Sanofi
Featuring articles: "A basis for innovation and continuous improvement of process understanding and control in pharmaceutical product development" by Magida Zeaiter, GlaxoSmithKline, "Flexible processing assures product quality" by Mark Morton, Phoenix Scientific Services and "Quality by design: A lifecycle concept for pharmaceutical analysis" by Joachim Ermer, Head of Quality Control…