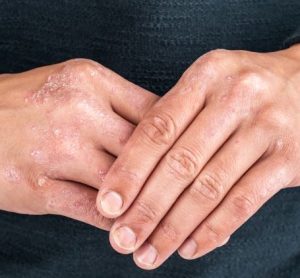
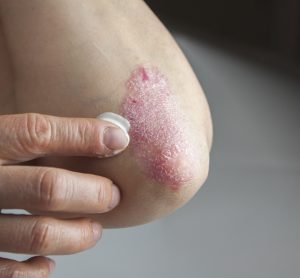
Investigational plaque psoriasis treatments show promise in late-stage trials
With the Phase III trials demonstrating sustained skin clearance in plaque psoriasis, this could lead to patients accessing more treatment options to manage the autoimmune inflammatory disease.