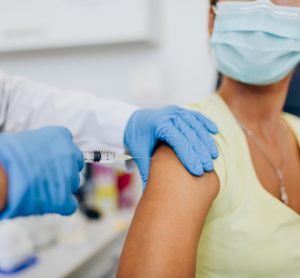
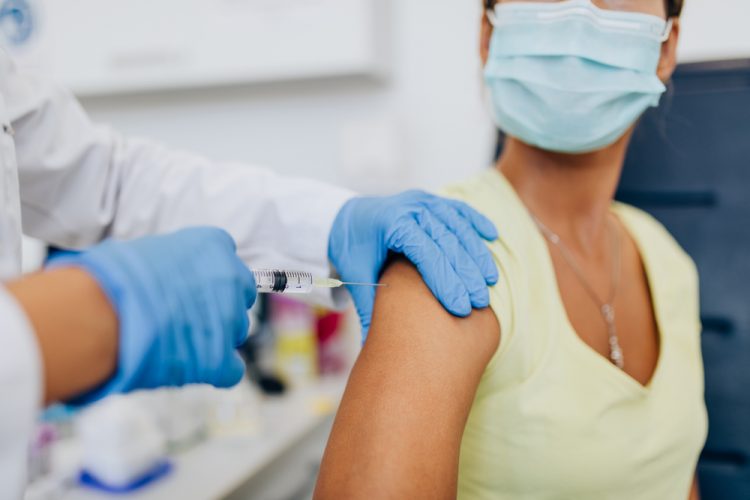
Regulators call for clinical research to include pregnant and breastfeeding women
The FDA and other global regulators are co-operating to address inadequacies in clinical research that leave pregnant and breastfeeding women lacking data on which to base their medical decisions.