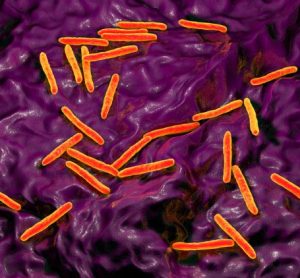
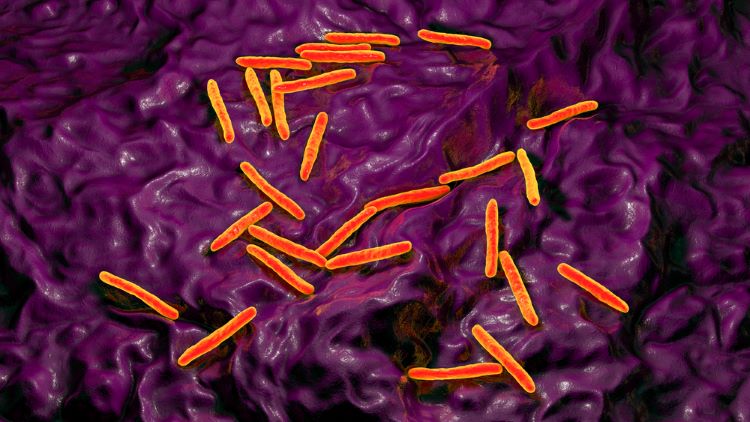
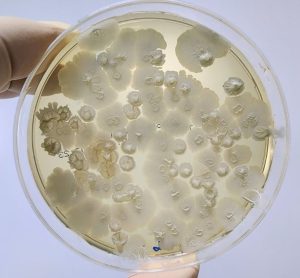
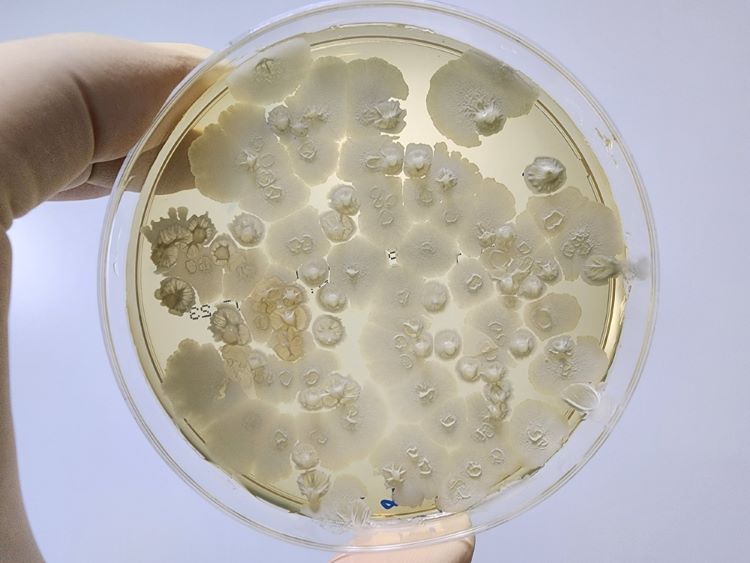
Alternative methods for mycobacterial testing in biological products
Considering the limited available information on implementing mycobacterial testing for quality control of biologicals, researchers have highlighted a suitable potential alternative detection method.