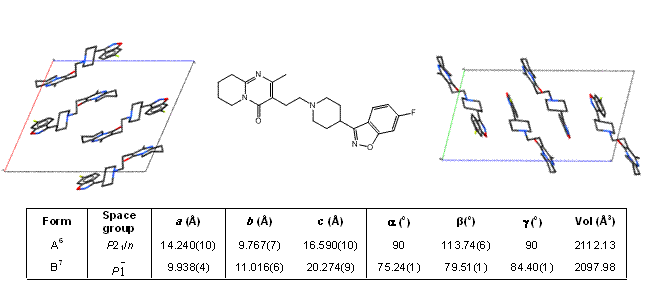
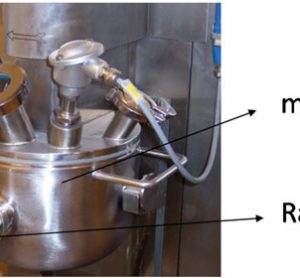
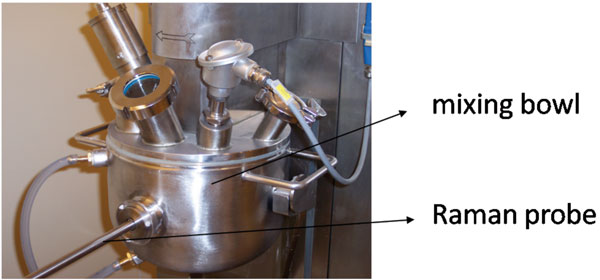
article19 August 2010 | By Andrew Riches, Professor of Experimental Pathology, School of Medicine, University of St. Andrews and Co-authors:
C. Simon Herrington, School of Medicine
Kishan Dholakia, Elisabetta Canetta, Antonia Carruthers, Michael Mazilu, Anna Chiara de Luca, School of Physics & Astronomy
Chris Goodman, Greg Kata, Nabi Ghulam, Kadi Nourdin, Department of Urology, Ninewells Hospital & Medical School, Dundee
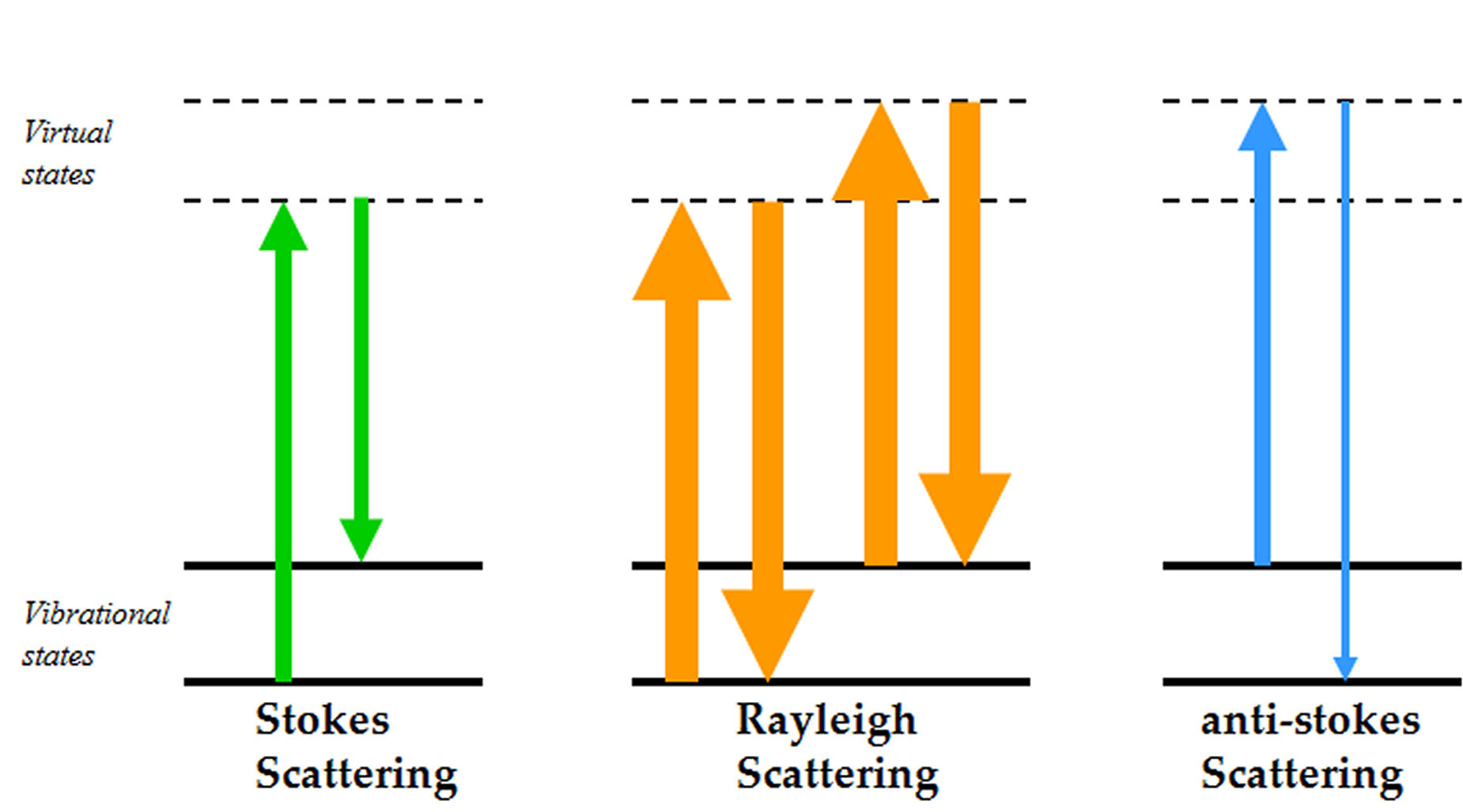
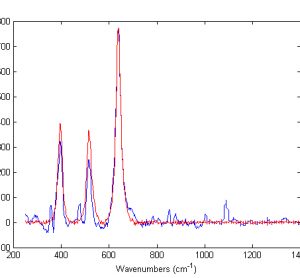
Raman spectroscopy has the potential to provide diagnostic information to the clinician. The technique has a number of advantages allowing individual cells to be interrogated without staining. With further developments in technology, the surgeon will be able to rapidly acquire accurate diagnostic information at the time of operation using fibre…