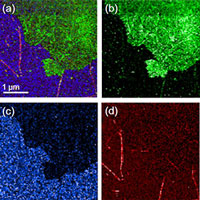
Application Note: Are you seeing the whole picture with Raman?
Raman spectroscopy offers a rapid, convenient method for positive identification of pharmaceutical products and their raw materials – on the manufacturing floor, in inventory, and at the shipping dock.