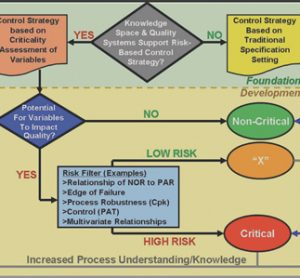
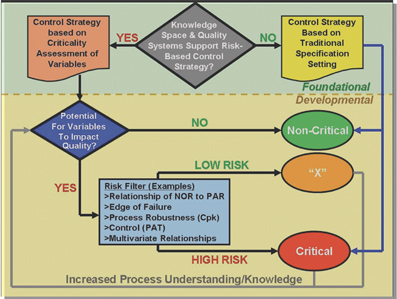
Pharmaceutical analysis in quality control
7 April 2008 | By
Pharmaceutical analysis in drug development mainly focuses on methods to identify and quantify potential new drug candidates, determine purity, identify by – products and degradation products in compatibility and stability studies, and to determine the drug substance’s fate in the organism. Challenging tasks like these require sophisticated techniques, dedicated equipment…