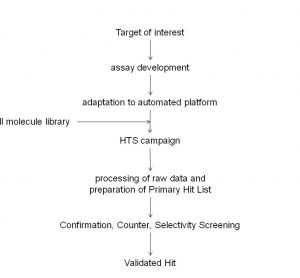
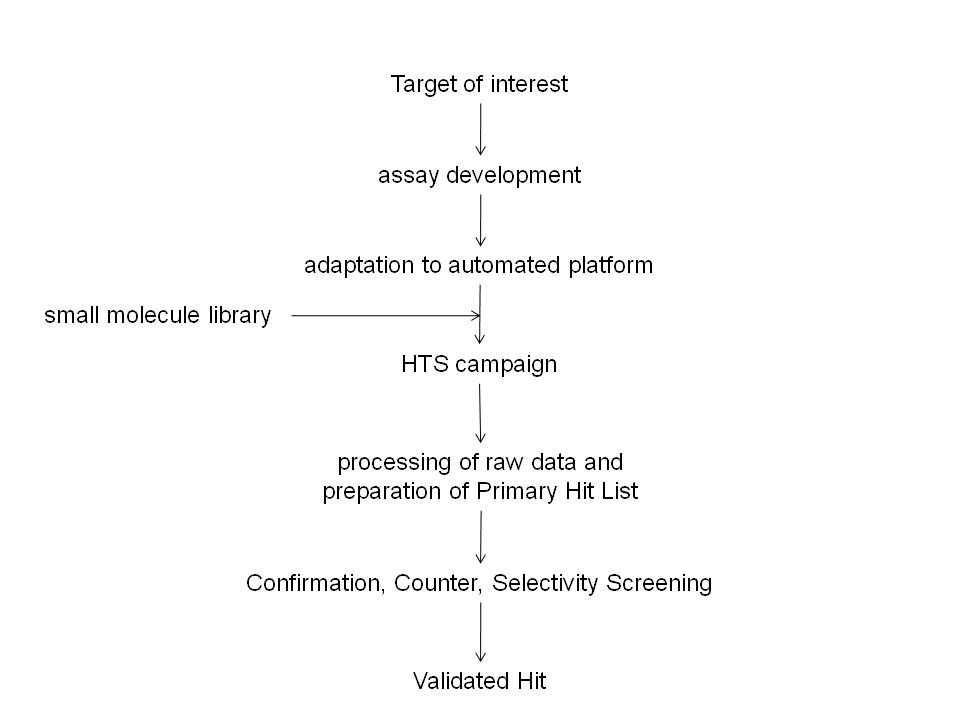
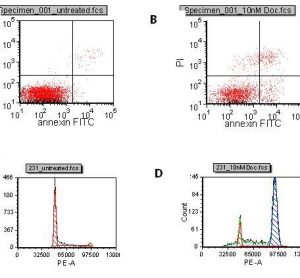
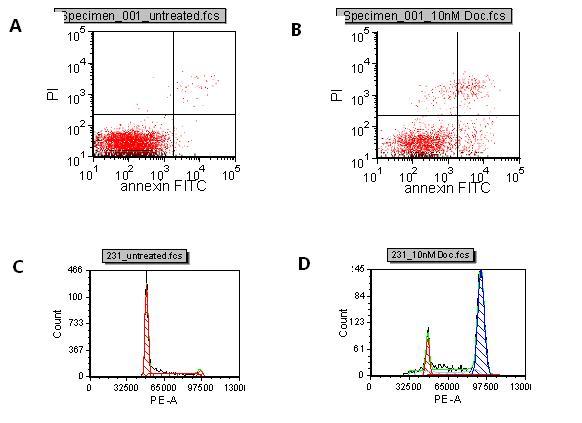
Thermo Fisher Scientific adds new Atlas CDS software control for Shimadzu Prominence HPLC
Thermo Fisher Scientific Inc., the world leader in serving science, today announced the availability of a new Thermo Scientific Atlas Chromatography Data System (CDS) instrument control software for the Shimadzu Prominence® and Prominence XR HPLC.