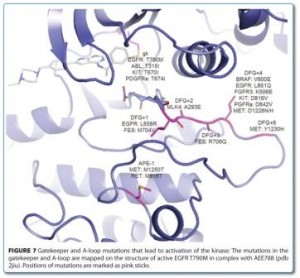
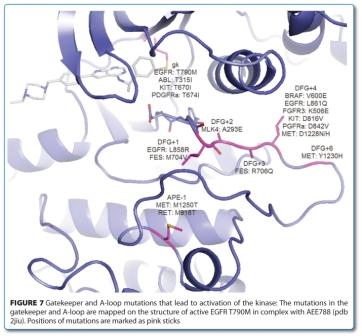
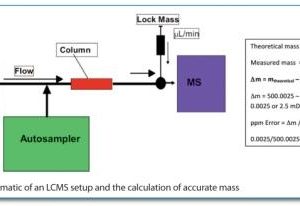
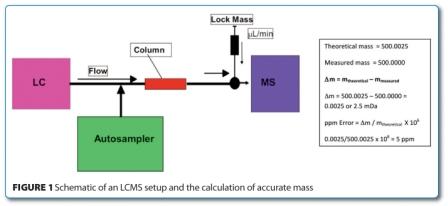
Conformational Bias: A key concept for protein kinase inhibition
28 February 2012 | By Henrik Möbitz, Global Discovery Chemistry, Computer Aided Drug Design, Novartis Institutes for Biomedical Research and Doriano Fabbro, Expertise Platform Kinases, Novartis Institutes for Biomedical Research
Protein kinases act as molecular switches with remarkable plasticity and dynamics upon interaction with specific regulatory domains as well as modulators. Conformation provides a conceptual framework for understanding many aspects of kinase biology. The kinase domain has precise structural prerequisites for signal transfer and can oscillate between two major conformations:…