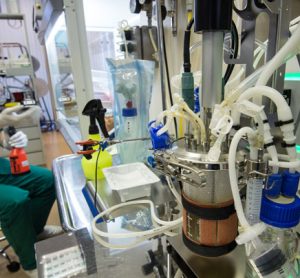
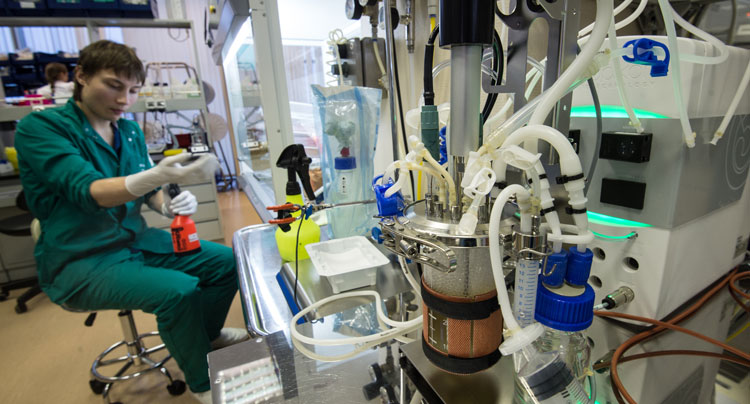
Is high resolution mass spectrometry the missing piece in continuous bioproduction?
Consistent with quality-by-design (QbD), and driven by the desire for continuous manufacture and real-time lot release, biopharmaceutical manufacturers require advanced process analytical technologies (PAT) for process monitoring and rapid analytical information generation.