Digitalisation of the clinical trial landscape
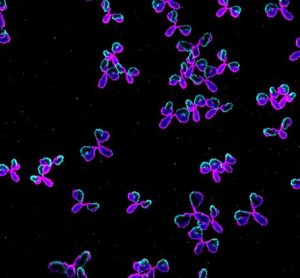
UCB Pharma's Chief Medical Officer Iris Loew-Friedrich, shares her perspective on digital innovation in clinical trials plus current challenges in clinical data management and how it could evolve in the future.