Towards a comprehensive open source platform for management and analysis of High Content Screening data
19 August 2010 | By Karol Kozak, Angela Bauch, Gabor Csucs,Tomasz Pylak & Bernd Rinn, ETH Zurich
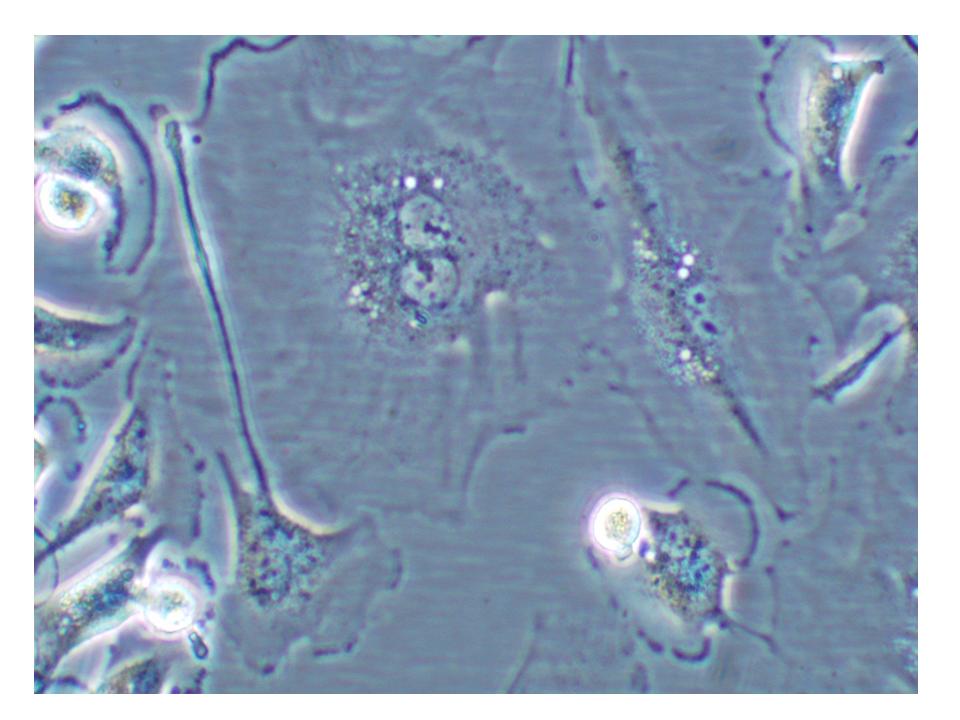
As High Content Screening (HCS) has moved into the mainstream for biological and pharmaceutical investigations, a lag of well integrated pipelines for automated acquisition, management and analysis of HCS results turns out to be a bottleneck for fully leveraging the wealth of information contained in a screen and moving to…