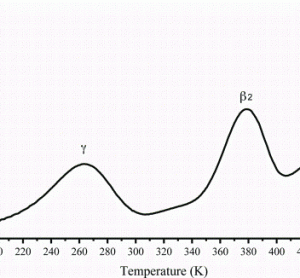
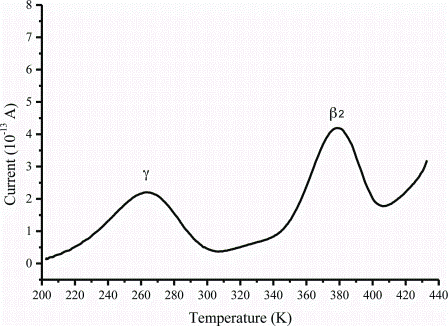
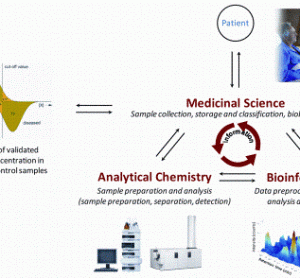
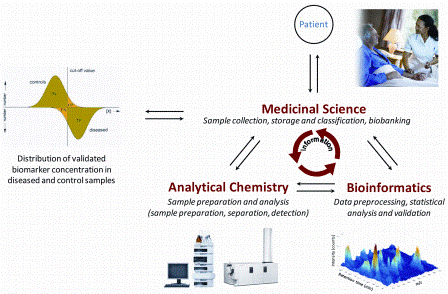
Integrating preclinical data into early clinical development
3 September 2012 | By Vikash Sinha, Clinical Pharmacology Leader, Janssen Research and Development
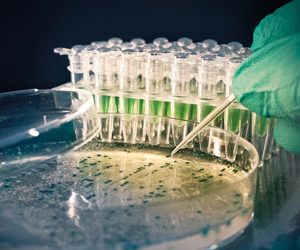
One of the important goals in preclinical and early clinical drug development is to reduce attrition rates and to improve our ability to pick winners and drop potential loser drug candidates. By being able to efficiently translate preclinical data and observations into possible clinical outcomes, one can make the drug…