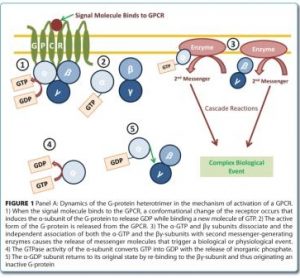
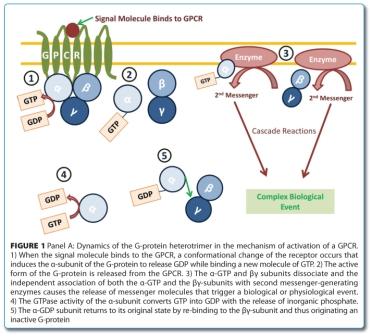
The need for proteomic-based biomarkers in the drug development pipeline
10 July 2012 | By Paul C. Guest, Department of Chemical Engineering and Biotechnology, University of Cambridge and Sabine Bahn Department of Chemical Engineering and Biotechnology, University of Cambridge & Department of Neuroscience, Erasmus Medical Centre
Pharmaceutical companies are under increasing pressure to improve their efficiency and returns on drug discovery projects. This is a daunting task considering that the average drug costs approximately one billion US dollars to develop and takes around 12 years from initial discovery to reach the market1. In addition, approximately 70…