Quality risk management and the economics of implementing rapid microbiological methods
Posted: 20 March 2009 | Dr Michael J. Miller | No comments yet
Quality risk management (QRM) is an important part of science-based decision making which is essential for the quality management of pharmaceutical manufacturing1. The ICH Q9 guideline, Quality Risk Management2 defines QRM as a systematic process for the assessment, control, communication and review of risk to the quality of drug product across the product lifecycle. Similarly, the FDA Final Report for Pharmaceutical cGMPs for the 21st Century – A Risk-Based Approach3, states that using a scientific framework to find ways of mitigating risk while facilitating continuous improvement and innovation in pharmaceutical manufacturing is a key public health objective, and that a new risk-based pharmaceutical quality assessment system will encourage the implementation of new technologies, such as process analytical technology (PAT), to facilitate continuous manufacturing improvements via implementation of an effective quality system.
The FDA’s PAT Guidance, which was finalised in 20044, describes a regulatory framework that will encourage the voluntary development and implementation of innovative approaches in pharmaceutical development, manufacturing, and quality assurance. Many new technologies are currently available that provide information on physical, chemical, and microbiological characteristics of materials to improve process understanding and to measure, control, and/or predict quality and performance. The guidance facilitates the introduction of such new technologies to improve efficiency and effectiveness of manufacturing process design and control, and quality assurance. A desired goal of the PAT framework is; therefore, to design and develop well-understood processes that will consistently ensure a predefined quality at the end of the manufacturing process, which is the foundation for the concept, that quality cannot be tested into products; it should be built-in or should be by design.
Sterile drug products are required to be free of microorganisms, and while a loss of sterility assurance can result in harm to the patient, the likelihood of detecting a sterility failure is low. Therefore, risk in sterile product manufacturing, especially aseptic processing, is relatively high when compared with other pharmaceutical processes, making risk management particularly important. Effective monitoring of aseptic manufacturing processes can help to ensure that a state of control is maintained (providing assurance of the continued capability of processes and controls to meet product quality), areas for continual improvement are identified (helping to understand and reduce process variability), process and product understanding is enhanced, and manufacturing agility and efficiencies can be realised (by reducing waste and wasteful activities, reduce lead time and increase manufacturing capacity)5. From a microbiology perspective, one can apply QRM principles in order to design a process to prevent contamination, investigate ways to correct a contamination event, and assess the potential impact of failing results on the patient6. Fortunately, recent advances in alternative microbiological monitoring platforms, such as real-time rapid microbiological methods (RMM), provide the analytical tools necessary to accomplish these tasks.
Many RMM technologies provide more sensitive, accurate, precise, and reproducible test results when compared with conventional, growth-based methods. Furthermore, they may be fully automated, offer increased sample throughput, operate in a continuous data-collecting mode, provide significantly reduced time-to-result, and for some RMM platforms, obtain results in real-time7. Most importantly, a firm that implements a RMM in support of aseptic processes may realise significant manufacturing efficiencies and a direct link to the QRM principles previously described, such as monitoring and controlling critical process parameters, reducing or eliminating process variability, and reducing the risk to patients. Additional benefits may include the elimination of off-line assays and a reduction in laboratory overhead and headcount (resulting in a decrease in the cost of product sold), lower inventories (raw material, in-process material, and finished product) and backorders, a reduction in warehousing space and associated costs, and a decrease in repeat testing, deviations, out-of-specification investigations and product rework, reprocessing or lot rejection8. Finally, recent discussions by the U.S. FDA have reinforced their desire to see an increase in the implementation of RMM technologies within the pharmaceutical industry. Dr. Brenda Uratani (CDER, FDA) described the benefits of using a RMM, and these included automating the testing process, electronic capture of test data and information creation, the ability to initiate investigations earlier as compared with conventional methods (CM), the reduction of risk associated with microbial contamination, and the use of the data as a continuum for process improvement9. Therefore, the implementation of the next generation of RMM represents significant progress toward the acceptance of microbiological PAT solutions for the industry, and is directly aligned with the expectations for pharmaceutical manufacturing, quality and operational excellence in the 21st Century.
Obviously, moving away from a CM to more modern RMM platforms has significant quality, regulatory and technology advantages. However, it is also important that a firm fully understand the economic benefits of carrying out this type of transformation. Therefore, a comprehensive economic analysis should be performed in order to justify the costs associated with the qualification and the implementation of these new technologies.
Recent presentations on this topic provide an overview of how an economic analysis for a RMM technology can be performed10,11. There are essentially three steps in developing a business case for introducing a new RMM:
- Review the existing CM and recognise potential technology, quality and business opportunities for implementing a RMM
- Identify available RMM platforms that will meet future technical and business needs
- Develop a business case for implementing a RMM including a financial comparison of the RMM and the CM that will be replaced
Review the CM and recognise new opportunities
Most conventional microbiological methods require long incubation times on agar surfaces or in liquid media in order to visually detect growth or colony forming units as an indication that microorganisms were present in the original sample. Additionally, confluent growth on agar plates may prevent individual organisms from being isolated, necessitating sub-culture onto additional agar media, delaying the final time to result even further. Furthermore, microorganisms that are stressed due to nutrient deprivation, or following exposure to sub-lethal concentrations of antimicrobial agents, such as preservatives, disinfectants, heat or decontaminating gases, may not replicate when cultured on artificial media, because the environment and incubation parameters are not truly optimal for the resuscitation and subsequent proliferation of organisms that may be present. These types of organisms are also known as viable but non-culturable, or VBNC. In the event these stressed organisms are able to replicate, the required incubation time to detect a positive response can be greatly lengthened. Unfortunately, by the time a positive result or out of specification count is obtained, which can be anywhere from a few days to over two weeks, the opportunity to respond to the excursion has long passed.
The impact of a contamination event on an existing manufacturing process could be significant, resulting in a potential hold on all products manufactured in the suspect area, not to mention shutting down a line or entire plant as lengthy investigations and retest strategies are initiated. For these reasons, the modern microbiology lab should look toward implementing alternative microbiology methods that can offer in-process, real-time microbiology testing with a broad range of pharmaceutical manufacturing applications. The use of rapid microbiological methods can assist our industry in facilitating progress to the desired future state of pharmaceutical manufacturing, with the ultimate goal of ensuring final product quality and improving manufacturing efficiencies.
Identify available RMM platforms that will meet future technical and business needs
A RMM can provide a significantly faster time to result with greater accuracy, precision, sensitivity and reproducibility when compared with a CM. Many RMM technologies are also very effective in detecting and quantifying VBNC or stressed organisms in the same time frame that the technology can detect healthy or uninjured organisms. Additionally, when information about the microbial control of manufacturing processes can be obtained in real-time, as is the case for purified water testing, in-process bioburden testing and environmental monitoring, a firm may be able to immediately respond to an out of specification finding or an adverse trend and minimise the impact to product and/or in-process material. Conventional methods cannot provide this level of monitoring and control12.
When selecting a RMM, it is important to understand the technical and business needs and benefits of implementing a RMM technology for its intended application. Technical benefits may include shorter time to result or results in real-time, greater accuracy, precision, sensitivity and reproducibility, single cell detection, enhanced detection of stressed and VBNC organisms, increased sample throughput and automation, continuous sampling, and enhanced data handling and trend analysis.
The business and economic benefits a firm may realise when implementing a RMM are numerous, and may include reduced testing time and testing costs for product release, reduction or elimination of off-line assays, laboratory overhead, resources and equipment, lower cost of product sold, decreased re-sampling, retests and deviations, reduction in rework, reprocessing and lot rejections, and a reduction in plant downtime.
A review of currently available RMM technologies should then be pursued, and when one or more technologies are identified that meet the technical and business needs/benefits for the intended microbiology application, a business case for implementing the new method should be developed.
Develop a business case and financial model
Developing a business case involves comparing the overall costs associated with a CM with the benefits and savings when implementing a RMM. This can be accomplished by first understanding all direct and indirect costs associated with the CM, followed by an understanding of the costs associated with the qualification and implementation of the new method, in addition to the savings and/or cost avoidances for the new method. Each of these financial components will then be used to economically justify the implementation of the new method.
In order to effectively develop the business case and financial model, a cross-functional team should be assembled that represents the various functions necessary to provide input with respect to the technical and business benefits of implementing the RMM. These may include Finance, Procurement, Quality Assurance/Quality Control, Regulatory Affairs, Manufacturing, Technical Services, Equipment Validation, Computer Systems Validation and Statistics.
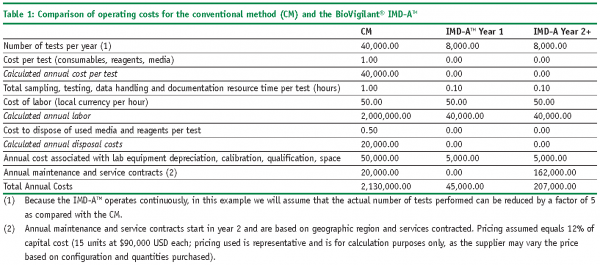
Examples of the costs and savings associated with the CM and the new RMM are as follows:
Costs associated with the CM:
- Cost per test (consumables, regents and supplies)
- Number of tests per year
- Total sampling, preparation, testing, data handling and documentation resource time per test (hours)
- Cost of labour including salary and benefits (local currency per hour)
- Cost to dispose of used media, reagents and consumables per test
- Laboratory equipment depreciation, calibration and qualification
- Overhead for laboratory and storage space
- Data management and record retention
- Preventive maintenance and service contracts for laboratory equipment
Costs associated with the RMM – same as for CM, but also include:
- Capital costs for initial investment
- Training
- System qualification and method validation costs
- Regulatory filing costs, if applicable
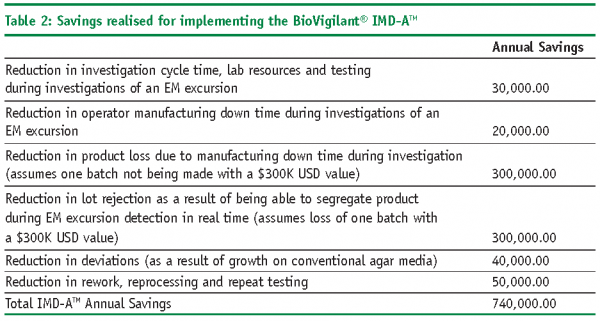
Savings associated with the RMM:
- Reduced testing cycle times
- Reduced finished product release cycle times
- Reduction in laboratory equipment and overhead
- Increased resource availability
- Reduced repeat testing and investigations
- Reduced lot rejection, reprocessing, rework
- Reduction in plant downtime
- Increased yields
- Reduced raw material, in-process and finished goods inventory holdings
When all of the elements associated with the costs and savings for both the CM and the RMM are understood, this information can be used to calculate whether there is a financial advantage for implementing the RMM. Different financial tools can be used to determine this information, including the Return on Investment (ROI) and Payback Period (PP).
ROI is the ratio of money gained or lost (realised or unrealised) on an investment relative to the amount of money invested. In this case, we are comparing the cost of performing the CM with the cost (and savings) of using a new RMM. The information is reported as a percentage (%) and usually represents an annual or annualised rate of return.
The PP is the time required for the return on an investment to “repay” the sum of the original investment. In the context of implementing a RMM, this would be the time (usually in years) required to realise enough cost savings/avoidances to pay for the initial investment of the RMM capital equipment and qualification/implementation activities.
Putting these concepts into practice is not a difficult task; however, a company’s financial department and/or purchasing or procurement groups should be consulted to assist in this economic exercise. Formulas for calculating the ROI and PP are readily available; however, the following models were recently presented during the PDA 3rd Annual Global Conference on Pharmaceutical Microbiology11 and were adapted for implementing a RMM:
Return On Investment (ROI)
ROI = Annual Net Benefits / RMM Investment
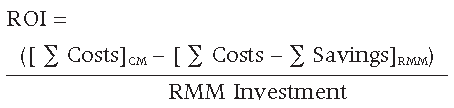
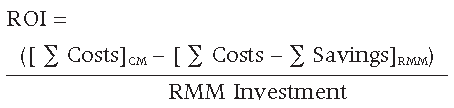
The ROI can be calculated for the first year (where the initial capital investment will be made) and then every year thereafter once the RMM is routinely used. The rate of return can take on any value greater than or equal to -100%. A positive value corresponds to an investment gain, a negative value corresponds to a loss, and a value of 0% corresponds to no change. Therefore, the higher the ROI number is, the greater the return the firm will realise on the initial investment for the RMM.
Payback Period (PP)
PP = RMM Investment / Annual Net Benefits
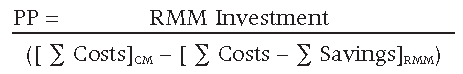
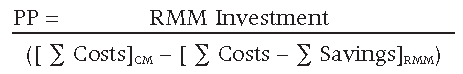
Calculating the PP will provide the time required to recoup the initial investment costs for implementing the RMM.
A case study in calculating the ROI and PP using a real-time RMM for environmental monitoring
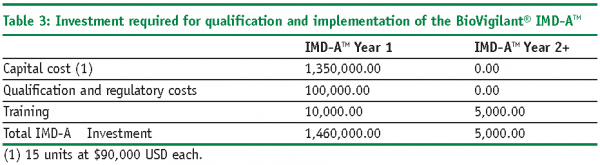
The following example illustrates how ROI and PP models can be used to economically justify the introduction of a RMM in place of a CM for environmental monitoring. In this case study, we will utilise the BoVigilant® IMD-ATM, a real-time and continuous viable and nonviable monitoring technology to replace agar-based active air sampling12,13. The IMD-ATM does not require the use of consumables, reagents or media, and eliminates the need for manual sampling and laboratory testing; therefore, we would expect to realise significant cost savings as compared with conventional, growth-based methods.
ROI Calculations
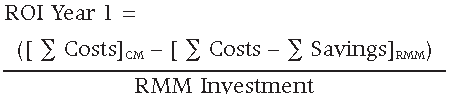
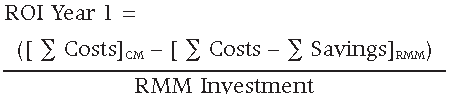
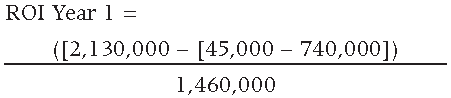
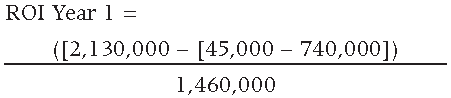
The resulting ROI for the first year is equal to 1.935 or 193.5%, resulting in a first year savings equal to $1,365,000 USD.
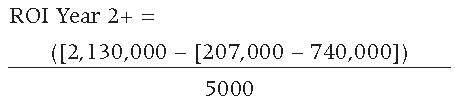
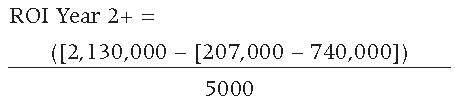
The resulting ROI for the second and subsequent years is equal to 532.6 or 53,260%, resulting in second year and subsequent annual savings equal to $2,658,000 USD.
The resulting ROI for a total of five years is equal to 9.231 or 923.1%, resulting in a five year savings equal to $11,997,000 USD.
PP Calculation
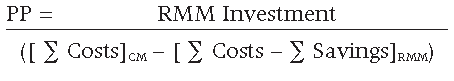
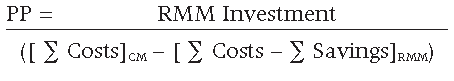
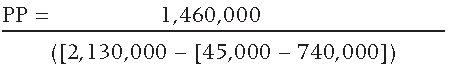
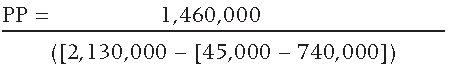
The resulting PP is equal to 0.52 years, or 6.2 months.
Summary
The case study provided herein provides sufficient economic justification for implementing the BioVigilant© IMD-ATM as an alternative to conventional active air sampling. Furthermore, the PP is relatively short due to a substantial cost savings during the first year of implementation. Although the scenario uses conservative numbers representative of a small parenteral fill-finish facility, the reader may realise a greater ROI when this model is applied to a large aseptic processing operation. The models presented in this manuscript may be used as written, or modified by the user as necessary, to develop their own economic assessment of implementing a RMM in place of current, conventional methods for environmental monitoring. The ultimate objective is to develop a robust financial assessment, coupled with a comprehensive technical and quality risk management strategy, to justify the qualification and implementation of a RMM that will provide assurance of continuous improvement and capability of aseptic processes, encourage manufacturing efficiencies and agility, and enhance the quality of drug products throughout their lifecycle.



References
- PDA. Quality risk management for aseptic processes. Technical Report #44. PDA J. Pharm. Sci. Technol. Suppl. 62(S-1). Parenteral Drug Association, Bethesda, Maryland: 2008.
- ICH. Quality Risk Management. International Conference on Harmonisation. Quality Guideline Q9. 2005.
- FDA. Final report for pharmaceutical cGMPs for the 21st century – A risk-based approach. U.S. Department of Health and Human Services, Food and Drug Administration, Rockville, Maryland. September 2004. Available at http://www.fda.gov/cder/gmp/gmp2004/CGMP%20report%20final04.pdf.
- FDA. Guidance for industry: PAT – A framework for innovative pharmaceutical development, manufacturing, and quality assurance. U.S. Department of Health and Human Services, Food and Drug Administration, Rockville, Maryland. September 2004. Available at http://www.fda.gov/cder/guidance/ 6419fnl.pdf.
- O’Sullivan, J. Evolving systems for product & process monitoring – An industry case study. FDA/PDA Co-Sponsored Conference Series on Quality Systems. Shanghai, China: 2008.
- Freidman, R. Risk management and regulatory considerations. PDA Risk Management and Aseptic Processing Conference. Bethesda, Maryland: May 16, 2008.
- Miller, M. J. Rapid microbiological methods in support of aseptic processing. In Practical Aseptic Processing: Fill and Finish; Lysfjord, J., Ed.; DHI Publishing, River Grove, Illinois and PDA, Bethesda, Maryland: 2009, in press.
- Miller, M. J. The impact of Process Analytical Technology (PAT), cGMPs for the 21st Century and other regulatory and compendial initiatives on the implementation of rapid microbiological methods. In Encyclopedia of Rapid Microbiological Methods Volume 1; Miller, M. J., Ed.; DHI Publishing, River Grove, Illinois and PDA, Bethesda, Maryland: 2005, 195-215.
- Uratani, B. Connecting science and regulation. 2007 PDA 2nd Annual Global Conference on Pharmaceutical Microbiology. Bethesda, Maryland: October 31, 2007.
- Miller, M.J. Points to consider when evaluating the return on investment (ROI) for the implementation of rapid microbiological methods. Rapid Microbial Methods, IBC Life Sciences, San Diego, California: 2004.
- Pascal Y. Rapid methods: return on investments. PDA 3rd Annual Global Conference on Pharmaceutical Microbiology. PDA, Chicago, Illinois: 2008.
- Uratani, B. and Smith, R.S. FDA perspectives on 21 CFR Part 11 requirements and its application in rapid microbiological methods. In Encyclopedia of Rapid Microbiological Methods Volume 1; Miller, M.J., Ed.; DHI Publishing, River Grove, Illinois and PDA, Bethesda, Maryland: 2005, 229-246.
- Jiang, J. P. Instantaneous microbial detection using optical spectroscopy. In Encyclopedia of Rapid Microbiological Methods Volume 3; Miller, M. J., Ed.; DHI Publishing, River Grove, Illinois and PDA, Bethesda, Maryland: 2005, 121-141.
- Miller, M.J. Case studies in the use of the BioVigilant IMD-A for real-time environmental monitoring during aseptic filling and intervention assessments in a manufacturing isolator. PDA 3rd Annual Global Conference on Pharmaceutical Microbiology. PDA, Chicago, Illinois: 2008.









