Emergence and identification of new designer drug products from the internet
Posted: 31 August 2011 |
Designer drugs represent a rapidly expanding phenomenon particularly facilitated by their internet availability. These drugs are continuously emerging as analogues of controlled substances (amfetamine, aminoindane, cathinone, phencyclidine, etc) and once an analogue has been banned, another replacement analogue appears on the market. They are often made in unlicensed laboratories which can result in their poor quality. This highlights the importance of analysing these products through detecting both their identity and purity. However, most of the analysis methods focused on emerging analogues of cathinone and very few studied other newer analogues such as phencyclidine derivatives. This is due partly to the regulations surrounding the analysis, the time consuming analytical procedures and the technical skills involved. Analysis of these designer drugs in the literature included both the identification of drug products and monitoring of the products consistency over a period of time. In all cases, the results showed that these products may contain a range of a single or mixture of components, including a designer drug, a pharmaceutical active agent, an excipient or inorganic material.
Designer drugs represent a rapidly expanding phenomenon particularly facilitated by their internet availability. These drugs are continuously emerging as analogues of controlled substances (amfetamine, aminoindane, cathinone, phencyclidine, etc) and once an analogue has been banned, another replacement analogue appears on the market. They are often made in unlicensed laboratories which can result in their poor quality. This highlights the importance of analysing these products through detecting both their identity and purity. However, most of the analysis methods focused on emerging analogues of cathinone and very few studied other newer analogues such as phencyclidine derivatives. This is due partly to the regulations surrounding the analysis, the time consuming analytical procedures and the technical skills involved. Analysis of these designer drugs in the literature included both the identification of drug products and monitoring of the products consistency over a period of time. In all cases, the results showed that these products may contain a range of a single or mixture of components, including a designer drug, a pharmaceutical active agent, an excipient or inorganic material.
Designer drugs represent a rapidly expanding phenomenon particularly facilitated by their internet availability. These drugs are continuously emerging as analogues of controlled substances (amfetamine, aminoindane, cathinone, phencyclidine, etc) and once an analogue has been banned, another replacement analogue appears on the market. They are often made in unlicensed laboratories which can result in their poor quality. This highlights the importance of analysing these products through detecting both their identity and purity. However, most of the analysis methods focused on emerging analogues of cathinone and very few studied other newer analogues such as phencyclidine derivatives. This is due partly to the regulations surrounding the analysis, the time consuming analytical procedures and the technical skills involved. Analysis of these designer drugs in the literature included both the identification of drug products and monitoring of the products consistency over a period of time. In all cases, the results showed that these products may contain a range of a single or mixture of components, including a designer drug, a pharmaceutical active agent, an excipient or inorganic material.
Designer drugs or so called ‘legal highs’ is a rapidly expanding phenomenon. These drugs emerge as analogues of controlled substances. As soon as a drug has been banned, another analogue appears on the market to circumvent any legal issues. Thus, they are often synthesised from a pre-existing publication, patent or research/data so they are a modification of an existing structure or class1.
The emergence of designer drugs began in the 1990s when drugs were being sold via the internet limited to derivatives of well-known classes including phenethylamines, tryptamines and piperazine derivatives2-6. Then in 2003 mephedrone emerged as the new recreational designer drug choice. Mephedrone is a synthetic cathinone, and has a similar structure to amfetamine7. Mephedrone was subsequently banned in the UK in April 2010, followed by the emergence of other cathinone derivatives on the market such as naphyrone8. Various other derivatives (both classic and novel) have appeared online including: aminoindanes, new phenethylamines, new tryptamines, phen – cyclidines, and cocaine alkaloids (Figure 1)9.
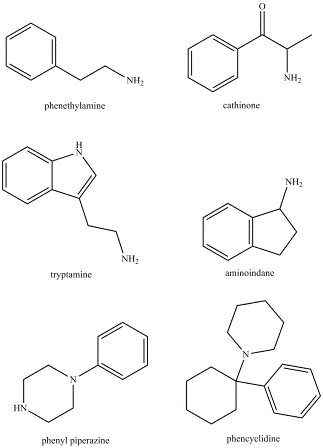

Figure 1 Chemical structures of the classes of designer drugs
The internet represents the main market forum for these drugs; thus they are mostly available from unregulated internet websites9,10. It has been documented that the websites for these compounds are increasing continuously11. What makes the situation more alarming is the attractive way these drugs are advertised over the internet, particularly to teenagers, because:
- they are promoted using attractive names particularly ‘suitable’ for the younger generation such as: head rush, meow meow, spice, doves original, exotic super strong, etc
- they are often advertised as ‘legal’ and thus perceived as ‘safe’ by the users
- they are accepted as a part of lifestyle rather than a misuse of drug
- they are not mentioned in the scientific literature and are relatively unknown to health professionals. Thus, according to one survey: 69 per cent of health professionals in the UK are seeing patients who are taking ‘legal highs’ and 57 per cent judged their knowledge of legal highs ‘poor’ or ‘basic’9
- they are often sold as something else such as mystical incenses, plant chemicals, research chemicals and bath salts12
- they are mentioned as not approved for human consumption and have unpre – dictable pharmacological actions and side effects
- they are easily purchased from internet websites with no restrictions.
These drugs are available mainly in the form of powders; however, they can also be presented in capsule or tablet forms. They are mostly purchased as single drugs, but mixtures of several designer drugs have been emerging lately on unregulated websites. A simple example was a tablet being sold as ‘Exotic ultra’, which consisted of a mixture of two cathinone derivatives (Figure 1): butylone and methylenedioxy- pyrovalerone (MDPV)8.
These drugs are typically synthesised in unlicensed laboratories10,12 by modifying the molecular structure of other controlled substances such as amfetamine, cathinone, ketamine, etc. The synthesis of these products is of poor quality10,12; thus, deliberate and/or accidental impurities might be identified in the purchased psychoactive substance. As a result, the product may not contain the drug as labelled and may contain another drug, an impurity, inorganic material or even a drug with no potential of abuse13-18. This highlights the importance of purchasing and analysing these products, reporting the findings and increasing public awareness of the health and social implications. In this respect, several questions need to be answered:
- Does the product contain the compound stated on the label?
- If not, does it contain another pharma – ceutical agent or excipient?
- Does it contain one drug only or a mixture of drugs?
- Does the drug contain any organic or metallic impurity?
- If the drug is a mixture, how much does it contain of each component?
However, the pace that these products appear on the website is faster than the time needed to order and analyse them. Table 2 shows the number of the new designer drug products analysed between April 2010 and April 2011. Whereas cathinone derivatives have been analysed, no products of other designer drug classes, such as phencyclidines, have been studied. This is due partly to the regulations surrounding the analysis, the time consuming analytical procedures and the technical skills involved.
Table 2: New derivatives of designer drugs analysed between April 2010 and April 2011.
| Class | Number of products | |
| Emerged | Analysed | |
| Phenethylamine | 6 | 0 |
| Tryptamine | 4 | 0 |
| Phenyl Piperazine | 1 | 1 |
| Cathinone | 11 | 8 |
| Aminoindanes | 5 | 1 |
| Phencyclidines | 6 | 0 |
Analysis of internet products
The methods in the literature focused on purchasing these compounds and identifying them using chromatographic, spectroscopic and spectrometric techniques. However, none looked at the amount of drugs these products contained.
One way of analysing these products is to order a labelled designer drug product from the Internet and confirm its identity or ‘label claim’13-15,19,20. In this respect, the identity of a mephedrone powder sample obtained from one internet website19 was confirmed using four different techniques: high resolution electrospray ionisation mass spectrometry (HRESI- MS), 1D and 2D NMR, elemental analysis and optical rotation (Table 1). The analysis showed that the sample contained mephedrone as follows. First, the 13C NMR showed the purity of the material, elemental analysis showed that the sample was in the form of salt. Second, the molecular modelling properties indicated that mephedrone was more polar than the amphetamine series. Thus, it needs higher doses to give similar effects. Third, the optical rotation showed that the ketone in mephedrone had a planar effect which contributes to the toxicity of naphyrone compounds.
Table 1: Details of the designer drugs products analysed.
| Product label | Drug/ Impurities found | Analysis technique(s) | Date |
| Cherries retropills2,3 | TFMPP/ Caffeine [17]. | GC-MS | 2010 |
| Cockstar2,3 | No psychoactive drug [17]. | GC-MS | 2010 |
| D2PM1 | 3FMC [8]. | GC-MS | 2010 |
| Diablos XXX extreme2,5 | Butylone [8]. | GC-MS | 2010 |
| Diablos XXX strong as hell2,3 | MBZP/ TFMPP/ CPP [17]. | GC-MS | 2010 |
| DMC1 | Caffeine/ lidocaine [14, 15]. | GC-EI/CI-IT-MS, NMR | 2010 |
| Dimethocaine1 | Dimethocaine [8]. | GC-MS | 2010 |
| Doves original2,3 | BZP/ TFMPP [17]. | GC-MS | 2010 |
| Doves red2.4 | MDPV [8]. | GC-MS | 2010 |
| Doves ultra2,4 | Butylone [8]. | GC-MS | 2010 |
| Exotic super extra strength2,3 | MBZP/ BZP/ DBP [17]. | GC-MS | 2010 |
| Exotic super strong2,3 | BZP/ TFMPP [17]. | GC-MS | 2010 |
| Exotic ultra2,5 | Butylone/ MDPV [8]. | GC-MS | 2010 |
| Giggle2,3 | pFPP [17]. | GC-MS | 2010 |
| Granules1,3 | Mephedrone [14, 15]. | GC-EI/CI-IT-MS, NMR | 2010 |
| Head rush2,3 | MBZP/ TFMPP/ CPP [17]. | GC-MS | 2010 |
| Hummer energy pills2,3 | Caffeine [17]. | GC-MS | 2010 |
| Jolly Green Granules1 | Mephedrone [8]. | GC-MS | 2010 |
| Lift2,6 | 3FMC/ Caffeine/ methylamine salt [21]. | GC-MS, NMR, FTIR | 2009 |
| Loved up retropills2,3 | pFPP/ Caffeine [17]. | GC-MS | 2010 |
| MDAI1 | Inorganic material [14, 15], mephedrone [14, 15], methylone [8]. | GC-EI/CI-IT-MS, NMR | 2010 |
| Mephedrone1 | Mephedrone [20]. | HR-ESI-MS, NMR, elemental analysis, optical rotation | 2010 |
| Neodove2,3 | caffeine/ mephedrone/ N-ethyl cathinone/ α-phthalimidopropiophenone [22]. | GC-MS, NMR, IR | 2007 |
| No label2,3 | 3-FMC/ Caffeine [17], caffeine [17],EC/ 4MMC [17], EC/ 4MMC/ Caffeine [17]. | GC-MS | 2010 |
| NRG-11 | 4-FMC/ pentylone/ MDPBP/ MDPV [16], butylone/ MDPV [14, 15], caffeine [14, 15], caffeine/ lidocaine [14, 15], caffeine/ mephedrone traces [14, 15], flephedrone [14, 15], flephedrone/ MDPV [14, 15], inorganic material [14, 15], mephedrone [8, 14, 15], naphyrone [14, 15], pentylone/ MDPBP [16], procaine/mephedrone traces [14, 15]. | GC-EI/CI-IT-MS, NMR | 2010 |
| NRG-2 | 4-FMC [8], 4-methyl-N-ethylcathinone [14, 15], benzocaine/ caffeine [14, 15], mephedrone [14, 15], mephedrone/ benzocaine [14, 15]. | GC-EI/CI-IT-MS, NMR | 2010 |
| NRG-31 | pentylone/ MPPP [16]. | GC-EI/CI-IT-MS, NMR | 2010 |
| Party on2,3 | Caffeine [17]. | GC-MS | 2010 |
| Pure bliss2,3 | Caffeine [17]. | GC-MS | 2010 |
| Rize 2 the occasion2,3 | No psychoactive drug [17]. | GC-MS | 2010 |
| Rocket fuel ultra2,5 | Butylone [8]. | GC-MS | 2010 |
| Space trips ultra2,5 | MDPV [8]. | GC-MS | 2010 |
| Space trips2,3 | MBZP/ TFMPP [17]. | GC-MS | 2010 |
| Spead freak ultra2,5 | MDPV [8]. | GC-MS | 2010 |
| Spirit2,3 | Mephedrone [22]. | GC-MS,NMR, IR | 2007 |
| Sub Coca 22,3 | α-phthalimidopropiophenone/ 2-fluoromethamphetamine [22]. | GC-MS,NMR, IR | 2007 |
| Sub Coca2,3 | caffeine [22]/ mephedrone/ N-ethyl cathinone/ α-phthalimidopropiophenone [22]. | GC-MS,NMR, IR | 2007 |
| Summer fusion elevate2,3 | Ephedrine/ Caffeine [17]. | GC-MS | 2010 |
| Super E retropills2,3 | TFMPP/ Caffeine [17]. | GC-MS | 2010 |
| Xtacy ultra2,5 | Butylone [8]. | GC-MS | 2010 |
| Xtacy2,3 | MBZP/ TFMPP/ CPP [17]. | GC-MS | 2010 |
| Xtacy2,3 | No psychoactive drug [17]. | GC-MS | 2010 |
| XXX strong as hell2,3 | BZP/ TFMPP/ DBP [17]. | GC-MS | 2010 |
1: The product is in the form of powder, 2: the product is in the form of tablet or capsule, 3: the product had no description of the content, 4: the product was labelled to contain amino acid blend, dicalcium phosphate and magnesium stearate, 5: the product was labelled to contain the component in 3 and ketone, 6: the product was labelled to contain flephedrone. 3FMC: 3-fluoromethcathinone, 4MMC: 4-methylmethcathinone, BZP: 1-benzylpiperazine, CI: chemical ionisation, CPP: chlorophenylpiperazine, DBP: 1,4-dibenzylpiperazine, DMC: dimethocaine, EC: ethcathinone, EI: electron ionisation, FMC: fluormethylmethcathinone, GC-MS: gas chromatogrpahy- mass spectrometry, Granules: the product was labelled to contain drug of the prolintane family, IT: ion trap, MBZP: 1-methyl-4-benzylpiperazine, MDAI: 3,4-methylene dioxy aminoindane, MDPBP: 3,4-methylenedioxy-α-pyrrolidinobutyrophenone, MDPV: 3,4-methylenedioxypyrovalerone, MPPP: 4-methyl-α-pyrrolidinopropiophenone, NMR: nuclear magnetic resonance, pFPPP: 4-fluorophenylpiperazine, TFMPP: 1-[3-(trifluoromethyl)phenyl]piperazine.
Similarly, the identity check of 24 designer drug products from 18 UK based internet websites (Table 1) was made13,14. The products were claimed to contain: NRG-1, NRG-2 and granules; however, a single drug or a mixture of designer drugs, anaesthetics, caffeine and inorganic material (Table 1) was found. The designer drugs identified included 4-methyl-Nethylcathinone, butylone, flephedrone, mephedrone, MDPV and naphyrone. The anaesthetics included benzocaine, lidocaine and procaine. The analysis was carried out using gas chromatography ion trap mass spectrometry (GC-IT-MS) in both chemical ionisation (CI) and electron ionisation (EI) modes and NMR spectroscopy. The GC method was able to resolve peaks from the products and the standards analysed. The MS chromatogram showed the characteristic α-cleavage of cathinone derivatives which leads to the formation of the immonium ion (CH2 = N+R2R3) and characteristic β-cleavage of the carbonyl group which resulted in the formation of the benzoyl ion. Table 3 shows the characteristic peaks in the MS chromatogram resulting from the cleavage of the cathinone derivatives. Confirmation of the presence of these compounds was made using NMR spectroscopy. In this respect, the two proton resonance indicated the substituents at C1 and C4 of the benzene ring.
Table 3: Characteristic fragmentations of designer drugs observed by GC-MS.
| Compound | Analysis mode | Characteristic ion | Peak m/z | Date |
| MDPV | EI/ CI | Immonium ion | 126/ 276 | 2010 [14] |
| Naphyrone | EI/ CI | Immonium ion | 126/ 282 | 2010 [14] |
| 4-methyl-N-ethylcathinone | EI | Immonium ion | 72 | 2010 [14] |
| Butylone | EI | Immonium ion | 72 | 2010 [14] |
| Flephedrone | EI | Immonium ion | 72 | 2010 [14] |
| Mephedrone | EI | Immonium ion | 72 | 2010 [14] |
| MDPV | EI | Benzoyl ion | 149 | 2010 [14] |
| Naphyrone | EI | Benzoyl ion | 155 | 2010 [14] |
| 4-methyl-N-ethylcathinone | EI | Benzoyl ion | 119 | 2010 [14] |
| Butylone | EI | Benzoyl ion | 149 | 2010 [14] |
| Flephedrone | EI | Benzoyl ion | 123 | 2010 [14] |
| Mephedrone | EI | Benzoyl ion | 119 | 2010 [14] |
| 4-fluoromethcathinone | EI/ CI | Immonium ion | 58/ 58 | 2010 [16] |
| Pentylone | EI/ CI | Immonium ion | 86/ 236 | 2010 [16] |
| MDPBP | EI/ CI | Immonium ion | 112/ 262 | 2010 [16] |
| MDPV | EI/ CI | Immonium ion | 126/ 276 | 2010 [16] |
| MPPP | EI/ CI | Immonium ion | 98/ 218 | 2010 [16] |
| Flephedrone | EI | Immonium ion | 58 | 2009 [21] |
| Flephedrone | EI | Fluorophenyl cation | 95 | 2009 [21] |
| Flephedrone | EI | Fluorobenzyloxy cation | 123 | 2009 [21] |
| 4-methylmethcathinone | EI | Immonium ion | 58 | 2007 [22] |
| 4-methylmethcathinone | EI | Benzoyl ion | 119 | 2007 [22] |
| α-phthalimidopropiophenone | EI | Immonium ion | 174 | 2007 [22] |
| α-phthalimidopropiophenone | EI | Benzoyl ion | 105 | 2007 [22] |
EI: electron ionisation, CI: chemical ionisation, 3,4-methylenedioxy-α-pyrrolidinobutyrophenone,
MDPV: 3,4-methylene-dioxy-pyrovalerone, MPPP: 4-methyl-α-pyrrolidinopropiophenone.
Similarly, three additional products obtained from two internet websites showed to have different identity from their label claim when identified by GC-EI/CI-IT-MS and NMR techniques15. These products were labelled as NRG-1 (two products) and NRG-3 (three products); but they showed to contain a mixture of cathinone derivatives. Thus, the NRG-1 product 1 consisted of a mixture of four cathinone derivatives: 4-fluoromethcathinone (4-FMC), pentylone, 3,4-methylenedioxy-α- pyrrolidinobutyrophenone (MDPBP) and MDPV. The GC method resulted in the separation of these four compounds at retention times of 6.73, 9.95, 11.12 and 11.53 minutes respectively. In addition, the NRG-1 product 2 contained a mixture of two cathinone derivatives: pentylone and MDPBP which were separated at retention times at 9.94 and 11.12 minutes. Also, the NRG-3 product 3 contained a mixture of two cathinones: 4-methyl-α-pyrrolidino – propiophenone (MPPP) and MDPBP which were separated at retention times at 9.39 and 9.94 minutes. The MS data of the cathinone derivatives present showed not only the immonium ion peaks (Table 3), but also the side chain specific to this ion. The NMR spectra indicated the coupling pattern of the drugs analysed and were consistent with the structures.
Other designer drug products were identified ‘from scratch’ as they had attractive names with no label claim of what they contained16,20-22. Thus four capsule products, obtained from one internet website, were shown to contain the presence of isomers of FMC and caffeine20. The four capsules were labelled as ‘Lift’, ‘Sub Coca Dragon’, ‘High Spirit’ and ‘Neo Dove’. The analysis of these products was made using GC-MS, 1H, 13C and 19F NMR and FT-IR (Table 1). The method was developed using the acetylated derivatives of pure compounds of three positional isomers of flephedrone: 2-FMC, 3- FMC and 4-FMC. The GC method resulted in the separation of these derivatives at 11.44, 11.46 and 11.66 minutes respectively. The MS spectrum showed peaks for the immonium ion, fluorophenyl cation and fluorobenzoyloxy cation (Table 3). The 19F NMR and FT-IR could identify these isomers easily. When the method was applied to the internet capsules, the GC-MS indicated the presence of both caffeine and fluoro-methcathinone. The direct 1H NMR confirmed the caffeine presence. Once this caffeine was washed with acetone, the resonance characteristic of fluoro meth – cathinone was observed.
In addition, four capsule products labelled as ‘Spirit’, ‘Sub Coca 2’ and ‘Sub Coca’ were shown to contain a mixture of three cathinone derivatives, an amphfetamine derivative and caffeine21. The cathinone derivatives were α-phthalimidopropiophenone, 4-methylmethcathinone and N-ethylcathinone and the amfetamine derivative was 2-fluoromethamfetamine (Table 1). The analysis was made using GC-MS, NMR and vapour and condensed phase infrared (IR)21. In this respect, the MS spectrum of both 4-methylmethcathinone and α-phthalidimidopropiophenone showed peaks characteristic to the formation of the immonium ions and benzoyl cations (Table 3). The NMR spectra of the 4-methylmethcathinone showed the 1H coupling pattern of two doublets at 7.86 and 7.35 ppm. This verified the 4-position of the methyl group attached to the phenyl ring. In addition, the 1H NMR of the α-phthalidimidopropiophenone showed an overlapping among the aromatic protons; however, the 13C NMR confirmed the two carbonyl resonances. The vapour and condensed phase of the 4-methyl meth – cathinone hydrochloride showed two characteristic peaks at about 2398 cm-1 and 1696 cm-1 indicating the amino and carbonyl groups respectively. On the other hand, the 2-fluoromethamphetamine was confirmed by the retention time in the mass chromatogram which appeared at 2.56 minutes. Also, the MS spectra of the N-ethyl cathinone was mistaken for N,N-dimethyl cathinone; however, derivatisation using pentafluoropropionic anhydride produced a small derivative peak that confirmed the presence if a secondary rather than a tertiary amine.
In addition, both the identity and consistency of 26 unlabelled designer drug products purchased from five internet websites over a period of six month was investigated using GC-MS16,22. The results showed that the products contained cathinone derivatives, piperazine derivatives, caffeine and drugs with no psychoactive effects (Table 1) as single or mixtures. However, there was a significant variation in the consistency in one quarter of these products over the assigned period of time.
The legality of 18 designer drugs products sold as





