Emerging NMR techniques as advanced tools for characterising biological parenteral products
Posted: 20 May 2019 | Bruce Yu - University of Maryland School of Pharmacy, Wei Xu - Merck & Co, Yongchao Su - Merck & Co | No comments yet
An important step in the research and development of biopharmaceuticals is to identify molecules with favourable physical and chemical stability profiles. Yongchao Su, Wei Xu and Bruce Yu discuss how solution, solid-state and benchtop nuclear magnetic resonance (NMR) methods can provide advanced biophysical characterisations of biological products.


Large molecules can exhibit complex structural behaviours due to their flexible molecular properties. An important step in the research and development of biopharmaceuticals is to identify molecules with favourable physical and chemical stability profiles, as these attributes are critical to the efficacy and safety of a drug product.1 In parenteral formulations, the conformational flexibility of proteins can result in different intra- and intermolecular contacts and sometimes trigger physical aggregation and chemical degradation. Thermal and mechanical stress encountered during the manufacturing processes, transport and storage of the finished products often introduce additional stability risk.
Biophysical characterisation plays an important role in the development and risk assessment of protein formulations. A wide array of analytical methods is necessary to probe the different aspects of the formulation, ranging from the molecular conformation to the higher order structure and the aggregated species. High-resolution characterisation tools are often required to determine structure, probe dynamics and study interaction in biological products. Particularly, nuclear magnetic resonance (NMR) methods have emerged as a powerful set of techniques for biophysical characterisation (Figure 1).
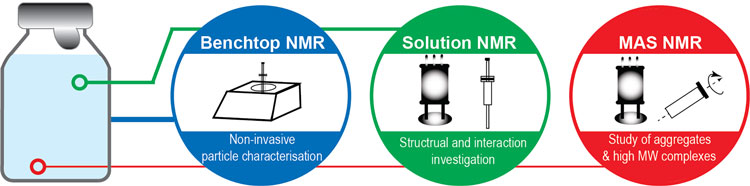

Figure 1: Advanced NMR techniques for characterising biologics formulation
High-field solution NMR as a versatile tool for in-depth biophysical characterisations
From a microscopic point of view, physical and chemical stability is governed by the structure of the molecular species in a biologics formulation. NMR characterises structures from Å to µm size and molecular dynamics from picoseconds to seconds, covering almost a full range of molecular behaviours in biologics products.2Compared to conventional tools, NMR is a versatile technique for investigating chemical and molecular structure, dynamics, and interaction at a high resolution and sensitivity.
In recent years, solution NMR has been explored by academia, biopharmaceutical industry and regulatory agencies to analyse biologics products. Successful examples include characterisations of physiochemical properties of biologics and mechanistic investigation of stabilising and destabilising interactions in parenteral products. For example, monoclonal antibodies (mAbs) represent the largest category of protein therapeutics on the market. One-dimensional (1D) NMR has been used extensively to identify and quantify the stability of mAbs.3-5It has facilitated investigation of structural and hydrodynamic profiles, the effects of excipients on self-association, and conformational change associated with aggregation in mAb formulations.
Solid-state NMR spectroscopy: predicting stability in lyophilised biological products…
Two-dimensional (2D) NMR methods enable examination of higher-order structure (HOS); describe the secondary, tertiary and quaternary structures; and probe the critical molecular properties of mAbs.6-8Fingerprint spectra can provide a sensitive spectroscopic identification and comparison on quality attributes of the drug substance over the time course of formulation development. Protein-excipient interactions have been studied to probe the mechanism of formulation instability at the molecular level9and screen stabilising excipients.10A significant number of NMR studies have focused on protein aggregation. For example, the particle size distribution is a critical quality attribute of drug products. Diffusion ordered spectroscopy (DOSY) NMR measures diffusion coefficients, which can derive the hydrodynamic radius for quantifying size distribution.11DOSY-NMR can also simultaneously report the particle size of the drug substance and excipients and identify protein-excipient complex in the formulation. Moreover, extensive efforts are made toward the mechanistic investigation of protein aggregation in studies of amyloid diseases.12The characterisations of prion protein oligomer, misfolding and effects of temperature, pH, metal ion and mechanical agitation in these studies offer opportunities for in-depth biophysical characterisations of large molecule formulations.
Solid-state NMR investigation of large biotherapeutic complexes and insoluble aggregates
In recent years, solid-state NMR (ssNMR) has been utilised to characterise a wide range of pharmaceutical materials including amorphous and crystalline dry powder, soft tissue specimen, fully-hydrated lipid systems, gel-like samples and insoluble protein complexes. In ssNMR experiments, samples are rotated at an angle (so-called magic angle spinning, MAS) with respect to the external magnetic field to average chemical shift anisotropies and dipolar interactions for narrower linewidth. As a cutting-edge technique, ultrafast MAS can spin up to 150kHz to generate 1H ssNMR spectra with significantly enhanced sensitivity and resolution. Another revolutionary technique, dynamic nuclear polarisation (DNP), can boost the ssNMR sensitivity by transferring high polarisation from electrons. Proton-detected techniques and DNP-enhanced ssNMR have opened new avenues in
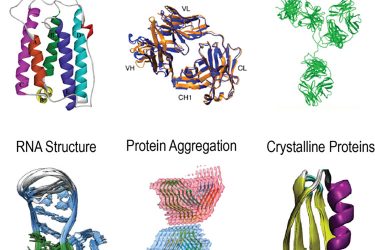

Figure 2: Solution and solid-state NMR characterisations of biopharmaceutical interesting molecular behaviours including protein-protein and protein-excipient interaction, RNA structure, higher order structure (HOS), mAb stability, protein aggregation and crystalline proteins.
biopharmaceutical applications.13 Many large protein complexes and insoluble aggregates experience slower molecular dynamics and thus fall into the motional regime for MAS NMR to study. Existing applications include large protein assemblies, RNA structure, fibrils and crystalline proteins (Figure 2). Taking protein aggregations, for example, insoluble particles represent a significant risk for biological formulation developments. Glucagon or glucagon-like peptides exhibit a strong tendency of fibrilisation and consequently physical instability. MAS NMR has been demonstrated as a powder tool for structure determination of disease-related amyloid fibrils.14,15 This powerful tool has immediate applicability for investigating insoluble aggregation of biological drugs.The goal is to solve the structure of undesired aggregation and identify the mechanistic cause of fibrillation. This approach can guide the rational design of the molecular motifs to improve the inherent stability of the development candidates. Besides insoluble aggregates, multidimensional MAS NMR is a primary tool for studying large protein complexes16 and can be employed for characterising protein-excipient and protein-protein assemblies in the formulation. Moreover, MAS NMR has also been used to determine RNA structures17 and shows great potential for investigating RNA-excipient complexes. In addition to the structural events in the sterile formulation, biological crystals present a new category of large molecular drugs. Bulk crystallisation provides one strategy for purification and requires solid-state structural analysis. Ultrafast MAS applications of micro- or nanocrystalline proteins have demonstrated successful examples of characterising large molecule drugs in the crystalline form.18
Time-domain benchtop NMR for probing biologics products
While high-field NMR techniques, whether solution or solid state, are apt to provide in-depth biophysical characterisations, they have limitations – such as expensive equipment with a large footprint,
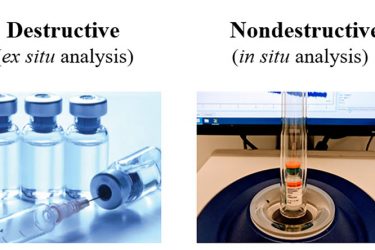

Figure 3: Benchtop NMR as a nondestructive for drug product characterisation
sophisticated and time-consuming operations, and high skillset requirements. Benchtop NMR presents a nondestructive biophysical tool for accessible and quantitative analysis, particularly at the drug product level. In the solid state, benchtop NMR has been used to analyse content uniformity of tablets19 and powder weight in vials.20 In the solution state, the water proton NMR signal has been used to detect mAb aggregates,21,22 insulin23and aluminium adjuvant24 filling levels. As illustrated in Figure 3, benchtop NMR also exhibits a great potential on quantitative analysis of aluminium-adjuvanted vaccines. Sedimentation of heterogeneous micron-sized particles in vaccines poses challenges to manufacturing. Benchtop 1H2O NMR can potentially serve as an in-line tool to monitor aluminium adjuvant suspensions without need of optical transparency or physical contact.
Conclusion
Solution, solid-state and benchtop NMR methods provide advanced biophysical characterisations of biological products for quantitative assessment, structural characterisation and mechanistic investigation.
Biographies






References
- Houde D, Berkowitz SA. Biophysical Characterization of Proteins in Developing Biopharmaceuticals, Elsevier, ISBN: 9780444595737
- Huang C, Kalodimos CG. Structures of Large Protein Complexes Determined by Nuclear Magnetic Resonance Spectroscopy, Annual Review of Biophysics, Vol. 46:317-336, 2017
- Chen K, et al. Simple NMR methods for evaluating higher order structures of monoclonal antibody therapeutics with quinary structure, J Pharm Biomed Anal. 2016 Sep 5;128:398-407
- Kheddo P, et al. Characterizing monoclonal antibody formulations in arginine glutamate solutions using 1H NMR spectroscopy, mAbs, 2016, VOL. 8, NO. 7, 1245–1258
- Poppe L, et al. Profiling Formulated Monoclonal Antibodies by 1H NMR Spectroscopy, Analytical Chemistry, 2013, 85, 9623-9629
- Brinson RG, et al. Enabling adoption of 2D-NMR for the higher order structure assessment of monoclonal antibody therapeutics, mAbs, 11:1, 94-105, 2019
- Arbogast LW. Mapping Monoclonal Antibody Structure by 2D 13C NMR at Natural Abundance, Anal. Chem. 2015, 87, 3556−3561
- Majumder S. Probing Conformational Diversity of Fc Domains in Aggregation Prone Monoclonal Antibodies, Pharm Res(2018) 35: 220
- Bis RL, et al. Role of benzyl alcohol in the unfolding and aggregation of interferon alpha-2a, Journal of Pharmaceutical Sciences, 104 (2015) 407-415
- Pandya A, et al. An Evaluation of the Potential of NMR Spectroscopy and Computational Modelling Methods to Inform Biopharmaceutical Formulations, Pharmaceutics2018, 10(4), 165
- Patil SM, et al. Comparison of NMR and Dynamic Light Scattering for Measuring Diffusion Coefficients of Formulated Insulin: Implications for Particle Size Distribution Measurements in Drug Products, AAPS J. 2017 Nov;19(6):1760-1766.
- Gilson MK, Radford SE. Protein Folding and Binding: From Biology to Physics and Back Again, Curr Opin Struct Biol. 2011 Feb; 21(1): 1–3.
- Su Y, Andreas L, Griffin RG. Magic Angle Spinning NMR of Proteins: High-Frequency Dynamic Nuclear Polarization and 1H Detection. Annual Review of Biochemistry. 2015. 84: p. 465-497.
- Su Y, et al. Secondary Structure in the Core of Amyloid Fibrils Formed from Human β2m and its Truncated Variant ΔN6, Journal of the American Chemical Society136 (17), 6313-6325
- Iadanza MG, et al. The structure of a β 2-microglobulin fibril suggests a molecular basis for its amyloid polymorphism, Nature communications9 (1), 4517
- Polenova T, et al. Magic Angle Spinning NMR Spectroscopy: A Versatile Technique for Structural and Dynamic Analysis of Solid-Phase Systems, Anal Chem. 2015 Jun 2; 87(11): 5458–5469.
- Yang Y, Wang S. RNA Characterization by Solid-State NMR Spectroscopy, Eur. J.2018, 24, 8698 – 8707
- Watt ED, Chad M, Rienstra CM. Recent Advances in Solid-State Nuclear Magnetic Resonance Techniques to Quantify Biomolecular Dynamics,Anal Chem. 2014 Jan 7; 86(1): 58–64
- Silva Elipe MV, Li L, Nagapudi K, Kook AM, Cobas C, Iglesias I, et al. Application of 19F time-domain NMR to measure content in fluorine-containing drug products. Magn Reson Chem. 2016; 54:531-538.
- Corver J, Guthausen G, Kamlowski A. In-line non-contact check-weighing (NCCW) with nuclear magnetic resonance (NMR) presents new opportunities and challenges in process control. Pharm Eng. 2005; 25:18-31.
- Taraban MB, DePaz RA, Lobo B, Yu YB. (2017) Water proton NMR: a tool for protein aggregation characterization. Chem. 89, 5494-5502.
- Taraban MB, DePaz RA, Lobo B, Yu YB. (2019) Use of water proton NMR to characterize protein aggregates: gauging the response and sensitivity. Chem. 91, 4107-4115.
- Briggs KT, Taraban MB, Wang W, Yu YB. (2019) Nondestructive Quantitative Inspection of Drug Products using Benchtop NMR Relaxometry: The Case of Novomix® AAPS ParmSciTech., submitted.
- Taraban MB, Fox CB, Yu YB. (2019) Assessing aluminum vaccine adjuvant filling, sedimentation, and resuspension in sealed vials using water proton NMR. Pharm. Rev.22(1), 70-73.
Issue
Related topics
Biopharmaceuticals, Drug Development, Drug Discovery, Nuclear magnetic resonance (NMR)








