Software tools that enable confident GC-MS analysis of extractables in pharmaceutical products
Posted: 30 August 2018 | Daniela Cavagnino | No comments yet
Pharmaceutical products come into contact with a wide range of polymeric materials on their journey from the production line to patients. Plastic and rubber contact surfaces are present at almost every stage of a product’s lifecycle: they’re present in single-use systems, such as filters and tubing employed in manufacturing processes; the packaging components that protect medicines during transport; and the delivery devices, such as syringes, pens and inhalers used to administer treatments to patients.

WHILE these materials are essential to ensure the sterility and quality of medicines during their manufacture and storage, they can also pose a serious risk to human health. Certain pharmaceutical products and packaging materials are incompatible and, if paired, can result in the leaching of potentially dangerous substances into products, possibly compromising the stability or, even worse, the pharmacological activity. These components, known as extractables and leachables, are the focus of rigorous testing workflows to ensure therapeutics are safe for use and meet regulatory requirements.
Extractables are chemicals that can be extracted by the pharmaceutical products from contact materials under accelerated laboratory conditions, such as elevated temperatures or aggressive solvents. Leachables are typically a subset of extractables that can migrate from packaging into a drug product under normal usage or standard storage conditions. Both extractables and leachables have the potential to react with an active ingredient or formulation excipients, potentially compromising the efficacy of the product and the dosage consistency, with serious implications for human health.
This article addresses the challenges facing these testing workflows, and how advanced software tools are helping to deliver more precise, confident analyses.
Extractables and leachables testing
Given the broad range of materials that may be present in a single device, packaging unit or storage container, identification of the contact component from which an extractable or leachable originates is essential. Plastics can contain a wide range of extractables and leachables derived from additives and storage aids such as antioxidants, plasticisers, emulsifiers and colourants. While information can be obtained from component manufacturers, given the complex supply chains involved in pharmaceutical manufacturing, robust testing workflows are required.
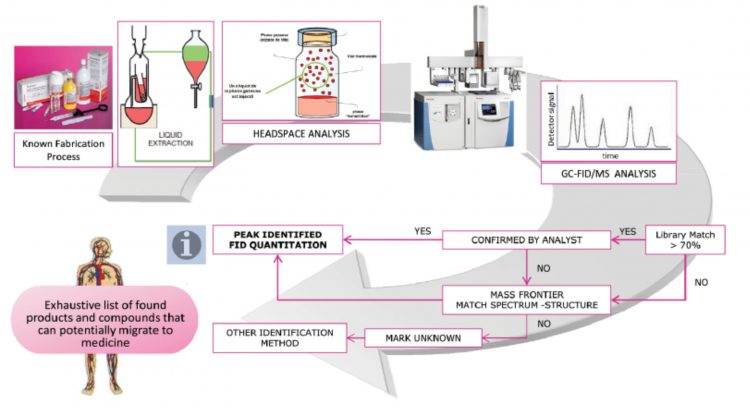

Figure 1: Typical workflow for extractables analysis
The identification of potential extractables typically involves a preliminary extractable study (Figure 1). This can be performed using a range of analytical methods, depending on the nature of the component under investigation. For the analysis of elemental composition, inductively coupled plasma analysis is typically used, while liquid chromatography mass spectrometry (LC-MS) is generally employed for the identification and quantitation of non-volatiles. For volatile components, gas chromatography mass spectrometry (GC-MS) using either direct headspace analysis or liquid injection following solvent extraction is typically employed.
A growing analytical challenge
The risk of polymer-derived extractables entering pharmaceutical products has increased in recent years due to the growing adoption of single-use technologies, novel packaging solutions and drug delivery systems. To protect patients and consumers from exposure to these components, regulatory bodies are demanding more information about drug contact materials and their potential to interact with pharmaceutical products, putting additional pressure on testing laboratories.
One of the biggest challenges associated with extractables testing workflows is identifying and quantifying compounds at very low limits of detection. A particular challenge relates to the large variation in potential dose between pharmaceutical products. An asthma inhaler, for example, may administer three or four doses of just 50 microlitres, while a dialysis bag may deliver a volume of several dozen litres. The ability to quantify potentially dangerous extractables at the trace level is therefore essential.
Fortunately, advances in instrument design and increasingly powerful data analysis packages are driving remarkable improvements in quantitative analysis. Using the latest spectral deconvolution software, analysts are able to overcome the challenges associated with background noise and possible analytical interferences, to produce very clean spectra from which to draw confident conclusions. Deconvolution programmes, such as the Automated Mass Spectral Deconvolution and Identification System (AMDIS), for example, can be used to extract ‘clean’ single compound mass spectra from a complex total ionic chromatogram (TIC) and match them with available mass spectral libraries for reliable identification.
This software operates in a three-step process. Firstly, the software counts the number of eluted compounds based on a minimum number of ions present at a common retention time. The corresponding mass spectrum is then extracted, and its contribution to the baseline and co-eluting mass intensities is eliminated. The software then checks against a user library to determine whether the target compounds are present, by simultaneously matching the retention time or retention index and mass spectrum. Finally, all the detected compound spectra are compared against the reference spectra of linked libraries. Various criteria can be used to filter the results; for example, isolating only the most abundant compounds in terms of peak area, or those with a minimum percentage area over a given value.
Deconvolution in this way enables the precise isolation of mass spectra even from co-eluting compounds. The ability to use an individual library of target compounds and combine retention time with mass spectral data makes it a powerful tool for analysis.
Detect the unexpected
With the increasing use of novel single-use components and innovative packaging, the potential for unknown extractables entering testing workflows is also set to rise. Determining the identity of compounds not present in commercial libraries was once a complex challenge, requiring a significant amount of time and a good deal of analytical detective work. However, advanced software solutions are helping testing laboratories identify unexpected compounds more quickly and confidently than ever before.
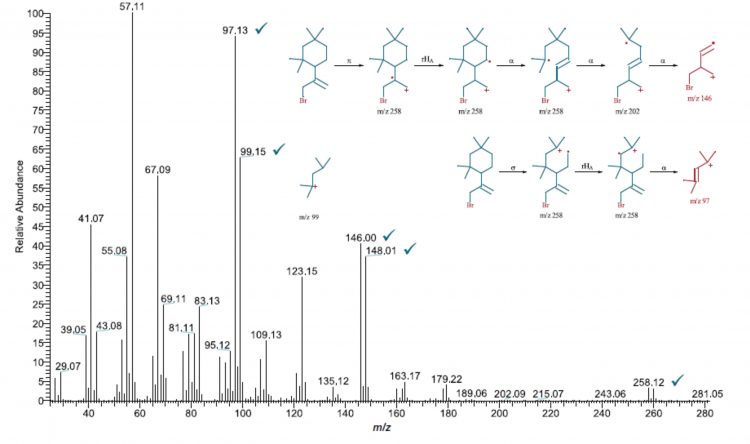

Figure 2: MS fragmentation pathway of an unknown compound in the isopropyl alcohol (IPA) extract of a plastic syringe, using Thermo Scientific Mass Frontier software (spectra obtained using a Thermo Scientific ISQ 7000 single quadrupole GC-MS system)
While acquired spectra may not fully match commercial library references, some matches may show structural similarities. These subtle clues as to the unknown compound’s identity can be used to piece together the full structure. Tools such as the Thermo Scientific Mass Frontier Spectral Interpretation Software can generate plausible proposals to explain the mass spectrum pattern for such an unknown compound by associating fragmentation pathways and ion structures with the unknown spectral pattern calculated using known fragmentation rules.
Figure 2 highlights how this approach can be used to provide a likely identification for an unknown compound extracted from a plastic syringe component using isopropyl alcohol. The proposed structures generated by the Mass Frontier software can well explain the mass spectrum pattern and fragmentation pathway, providing a valuable tool for unknowns elucidation.
Parallel detection using full-scan mass spectrum and Gas Chromatography-Flame Ionisation Detector (GC-FID) showed well correlated chromatographic patterns, obtained using a Thermo Scientific TRACE 1310 GC system. In this way, after identification of compounds, routine analysis can be performed reliably using the GC-FID, as an easy-to-use screening solution.
Conclusion
Extractables and leachables found in polymer-based single-use technologies, packaging components and drug delivery systems present a serious health hazard that demands robust safety testing protocols. The ability to accurately identify and quantify known and unknown extractables in these materials is therefore essential to safeguard human health. Thanks to powerful GC-MS instrumentation and the software solutions used to investigate this data, analysts can cut through the complexity of extractables testing workflows and ensure pharmaceutical products are safe for the patients who need them.
_______________________________________________________________________________
BIOGRAPHY
DR DANIELA Cavagnino received a Master’s Degree in Chemistry from the University of Milan, Italy. She started her career in gas chromatography at Thermo Fisher Scientific, spending several years in the R&D laboratories working on GC technology innovation. She then conveyed her technical background into product management and marketing management roles, and she now has more than 10 years of experience promoting GC/GC–MS technology and applications in several different market segments. During her career, she has participated as an author and co-author in technical publications and as a lecturer at international conferences.
The rest of this content is restricted - login or subscribe free to access


Why subscribe? Join our growing community of thousands of industry professionals and gain access to:
- bi-monthly issues in print and/or digital format
- case studies, whitepapers, webinars and industry-leading content
- breaking news and features
- our extensive online archive of thousands of articles and years of past issues
- ...And it's all free!
Click here to Subscribe today Login here
Issue
Related topics
Analytical techniques, Biopharmaceuticals, Mass Spectrometry, QA/QC, Research & Development (R&D)









