Specificity in the recombinant factor C test for endotoxin
Posted: 26 April 2018 | Kevin L Williams - bioMerieux | No comments yet
Limulus amebocyte lysate (LAL) users, compendial experts and regulators are still orienting themselves to the recombinant factor C (rFC) assay. Changes to compendial standards do not occur overnight and, for now, users willing to change must perform the alternative validation procedure USP <1225>.


WHEN validating an alternative assay, the specificity of the assay for the impurity to be detected is a requirement. The test must be able to distinguish the impurity in the sample from other, non-related substances and impurities. With the recombinant version of the horseshoe crab biosensor, rFC, there are three levels of specificity provided compared with LAL testing. This paper seeks to highlight three different specificities of rFC that include (i) Enzymatic, (ii) Spectral, and (iii) Genetic level specificity.
Level 1. Enzymatic specificity
The molecular interaction of the factor C biosensor with endotoxin is an ancient enzymatic specificity developed in some invertebrates1 in which the zymogen protein interacts with and binds endotoxin in the region of factor C sushi peptides2. This binding then facilitates the breaking of a specific bond at the serine protease end (the opposite end) of the zymogen. The factor C molecule has now become ‘activated’ through its interaction with endotoxin and, in this new form, is specific for the next protein in the serine protease cascade (factor B). In the case of the recombinant protein, the activation of the zymogen reacts with a small fluorophore peptide of a specificity that mimics factor C’s interaction with factor B (Figure 1). This refers to enzymatic activation of factor C zymogen by endotoxin.
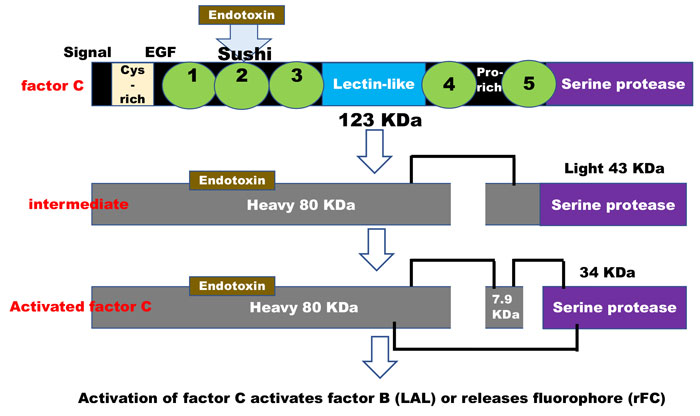

Figure 1: Activation of factor C activates factor B (LAL) or releases fluorophore (rFC)
Derived from references 2 and 3: “During the lipopolysaccharide-mediated activation of factor C, its single-chain form is converted to a two-chain intermediate form with an 80- kDa heavy and a 43-kDa light chain, and the light chain is subsequently cleaved at a unique Phe-Ile linkage to form a 7.9-kDa A chain and a 34-kDa B chain held together with a disulfide bond(s). The resulting three-chain factor C shows an ability to activate factor B and to hydrolyse a synthetic tripeptide substrate, Boc-Val-Pro-Arg-NH-Np.”4
It has been known for some time that LAL contains an alternative enzymatic pathway that can be activated by fungal and cellulosic breakdown byproducts, called the beta-glucan pathway. Non-endotoxin LAL reactive materials (LRM) in drug raw materials and products caused considerable concern upon the initial discovery of the additional pathway.5,6 As Figure 2 shows, beta-glucan acts upon the proclotting enzyme rather than factor C. Thus, LAL is not specific for endotoxin, whereas recombinant factor C assay is specific only for endotoxin. LAL users can use a beta-glucan blocking buffer to create an LAL test that is specific for endotoxin,7 see Figure 2.
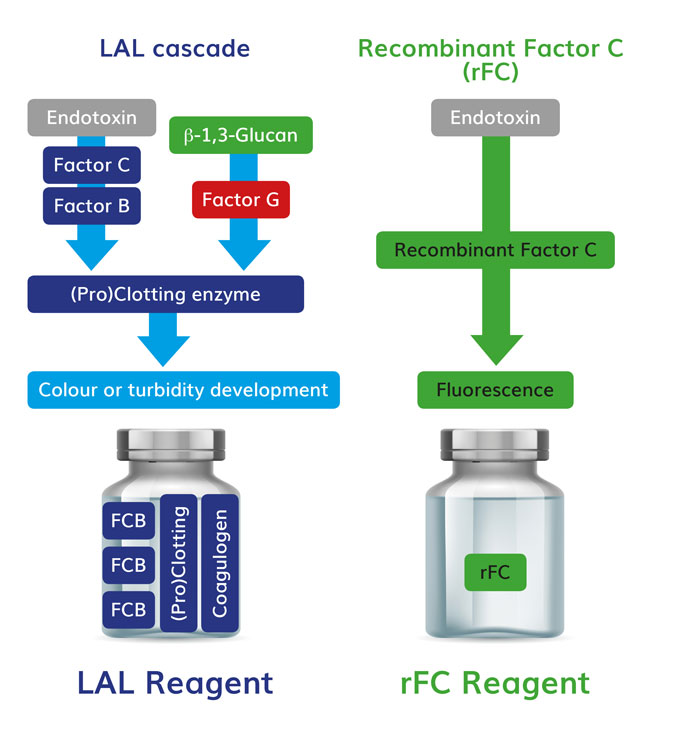

Figure 2: The simplification of the protein test milieu provides the first level of specificity for endotoxin testing via rFC
Level 2. Spectral specificity
Specificity here explores the fluorescent spectrophotometric detection method used with recombinant factor C assays. Various non-specific colour changes can occur with absorbance-based test methods (colourimetric and turbidimetric), whereas the fluorescent method used by recombinant factor C employs a very specific fluorophore with very specific excitation and emission wavelengths. In fluorescent assays, the excitation of a sample using a specific wavelength (380nm) and a different specific wavelength for emission detection (445nm) provides another level of specificity in performing the assay. Thus, random interference in terms of colour change, light ingress or other chemical change related occurrences are invisible to the fluorescent test.
Absorbance tests have been used successfully for several decades in LAL chromogenic and turbidimetric assays. But for any given, specific sample one may need to troubleshoot why a consistent assay cannot be obtained. Geisler lists some of the factors that can affect absorbance assays.
- Selectivity: UV / Vis spectrophotometer does not discriminate between the sample of interest and contaminants that absorb at the same wavelength
- Stray light: the detectors used in spectrophotometers are broadband, meaning they respond to all the light that reaches them. If there are impurities in the sample that reflect light, an erroneous reading may be recorded. Stray light also causes a decrease in absorbance and reduces the linearity range of the instrument
- Sample conditions: absorption results can be influenced by temperature, pH, impurities and contaminants. All these factors can change the absorption properties of the sample, leading to inaccurate readings.
Geisler also outlines the general advantages of fluorescent methods.
- Sensitivity: the sensitivity of fluorescence detection is approximately 1,000 times greater than absorption spectrophotometric methods
- Specificity: only molecules that fluoresce are detected by this method, resulting in greater specificity compared with UV / Vis absorption
- Wide concentration range: fluorimetry generally can detect more than three to six log orders of concentration without sample dilution or modification of the sample
- Accurate results: the sensitivity and specificity of fluorescence measurement leads to potentially more precise and accurate readings.
The rest of this content is restricted - login or subscribe free to access


Why subscribe? Join our growing community of thousands of industry professionals and gain access to:
- bi-monthly issues in print and/or digital format
- case studies, whitepapers, webinars and industry-leading content
- breaking news and features
- our extensive online archive of thousands of articles and years of past issues
- ...And it's all free!
Click here to Subscribe today Login here









