A paradigm change in biologics microbiological contaminant control
Posted: 17 October 2017 | Kevin L Williams | No comments yet
Contaminant detection methods derived from the innate, proinflammatory responses of rabbits and Limulus blood have been used to determine the existence of microbial artefacts in small-molecule drugs (SMDs) and large-volume parenterals (LVPs) for decades…


Even with the advent of biologics, the historical paradigm of microbiological control has largely continued on this path. The testing of artefacts that more specifically activate the adaptive immune system without setting off proinflammatory responses has taken place only recently. However, in terms of the ability of microbial and microbial mimetic artefacts to activate the adaptive immune system, biologics are different.1
Beyond size and complexity, biologic molecules differ from SMDs in that they are grown in reactors using living organisms rather than being chemically synthesised. Thus, any impurities that remain from the expressions system (carbohydrates, yeast, E coli, plants, etc) must be removed. Also, the processing streams post protein recovery are not sterile and consist of pH-neutral solutions, so downstream hold-time studies are important to ensure that bioburden and endotoxin cannot exceed limits.2
Biologics are also different because they trigger immunological side effects that SMDs and LVPs do not. A simple fever response in an SMD does not progress to antibody production against the small molecule drug and in this way, historically, fever always indicated contamination from endotoxin. In contrast, Bald3 describes the infusion reaction associated with biologics that commonly include fever, thus:
“Infusion of many biologics, particularly mAbs, provokes a characteristic infusion syndrome, usually within one or a few hours during/after the first administration. Whereas most reactions are mild to moderate with symptoms often described as ‘flu-like’, with fever, chills, rigours,4 headaches, nausea, asthenia, rash, and pruritus, a small number of patients, mostly at the first or second infusion, show potentially fatal symptoms …”
Endotoxin administration (infusion) elicits a very similar reaction in humans: “Endotoxin doses of 2-4ng/kg body weight cause flu-like symptoms (fever, chills, myalgia, headache, nausea) …”5
Pharmacovigilance for biologics is not what many assume. The FDA’s reporting mechanism is MedWatch,6 which is a voluntary system and as such cannot be relied upon for statistical determination of events. The Code of Federal Regulations (CFR)7 requirement that drug companies report adverse responses to the FDA does not cover responses that are expected as per package inserts. For this reason, fever is not considered an adverse drug response under the CFR definition.
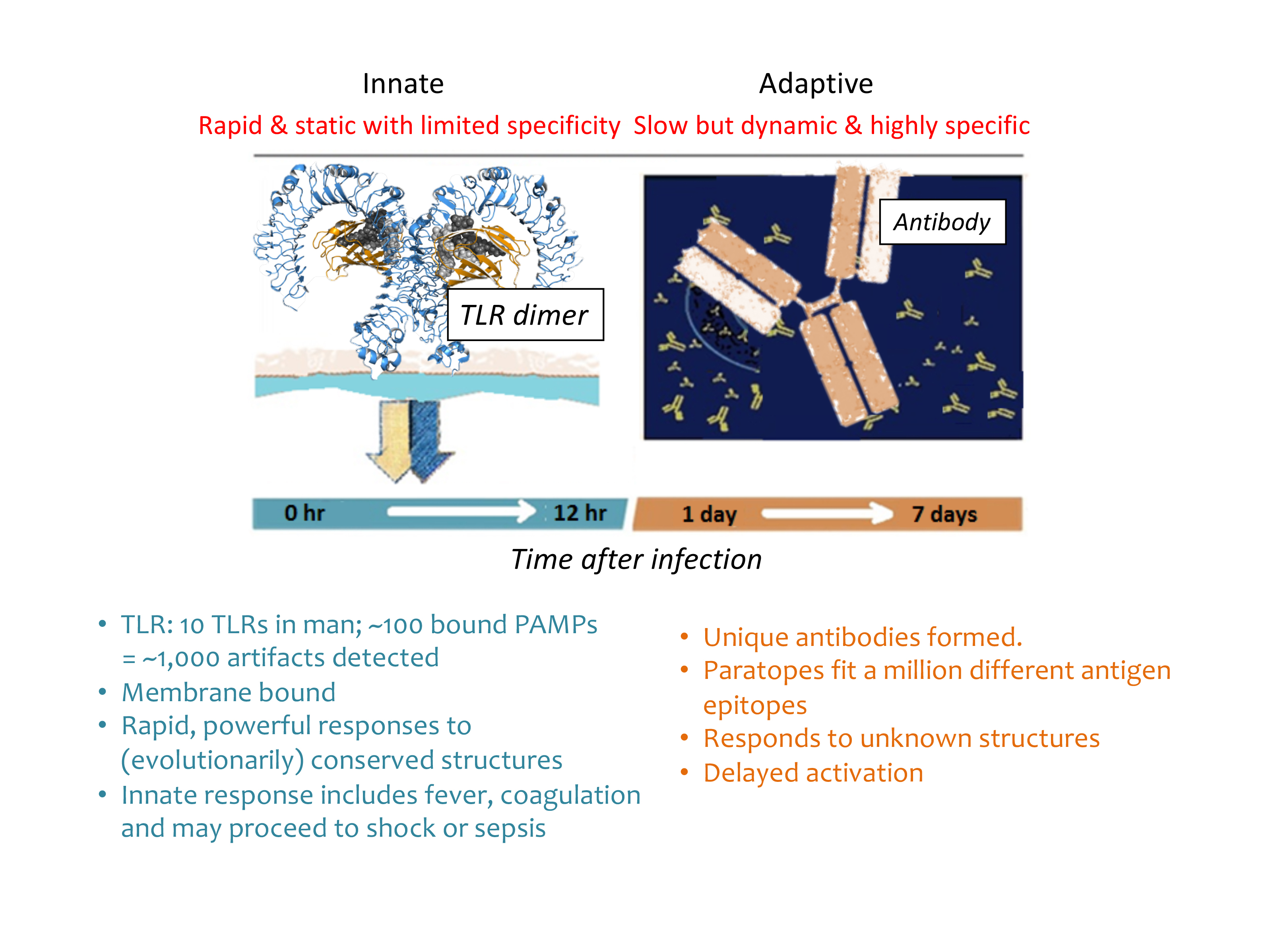

Figure 1 Figure1: Mammalian-microbial interfaces of innate and adaptive immune systems. Around 10 TLRs in man and around 100 bound MAMPs = around 1,000 artefacts detected. Rapid, powerful responses to (evolutionarily) conserved structures. Antibody paratopes fit up to a million different antigen epitopes. Responds to unknown structures after delayed activation. MAMPs > TLRs > cytokines > (B and T lymphocytes) > plasma cells > antibody production.
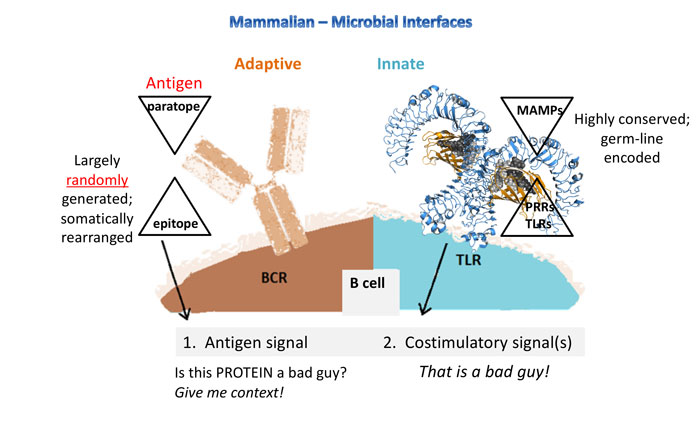

Figure 2: Whereas the innate response is fast and forewarned (germ-line encoded receptors), the adaptive response is created from largely random, somatic arrangements and is ‘guessing’ to match antigen structures. Why is this important? It puts the weight for antibody production back onto the activation of TLRs and LPS is the most potent and ubiquitous TLR activator.
The potential for immune responses gives biologics control a different context. Some artefacts that are not proinflammatory have the capacity to stimulate the immune system. See Figure 1 and Figure 2 for an overview of the major microbial interfacing architecture of adaptive and innate immune systems. The earliest indication that biologics control would proceed by different rules dominated by immune responses was seen in the effort to gain greater control of microbial mimetics8 including aggregates and particulates,9 as well as emulsions.10 Detection methods have accumulated for biologics and now include host cell proteins (from expression systems)11 and host cell DNA/RNA.12,13 In one instance, flagellin (a protein polymer making up flagella) co-purified with a drug substance caused adverse clinical responses.14 β-glucans have also been implicated as immune modulating microbe-associated molecular patterns (MAMPs), even though they have no overt proinflammatory effects.
Endotoxin as model immune stimulant
Endotoxin provides a good example for studying the difference between proinflammatory and immunostimulatory effects. Endotoxin in low doses, or from low potent forms of bacterial LPS,15 masked or detoxified,16 can be immunostimulatory without an associated pro-inflammatory activation. Detoxification is a word borrowed from vaccinology which has over 100 years of science that is largely not accessed by pharmaceutical scientists. Endotoxin is used as a vaccine adjuvant to stimulate the immune system in order to make microbial or viral proteins immunogenic for vaccination purposes. This has not been lost on FDA researchers17,18 and regulators19 who have studied the ‘adjuvant effect’ and determined that non-proinflammatory (or non-toxic) lipopolysaccharide (LPS) retains its ability to stimulate the adaptive immune system and promote the formation of anti-drug antibodies (ADAs). Such unwanted immunogenicity is a bane to biologics drugs.
Endotoxin advances a two-pronged attack in mammals (Limulus does not have an adaptive immune system):
- Proinflammatory innate response that is rapid and powerful, evidenced in fever and coagulopathy and which may proceed to shock or sepsis
- An immunostimulative response that is delayed (four to seven days) and more difficult to directly associate with LPS and other MAMPs, but which ends in an adaptive response that, ultimately, can include B-cell production of antibodies against therapeutic proteins.
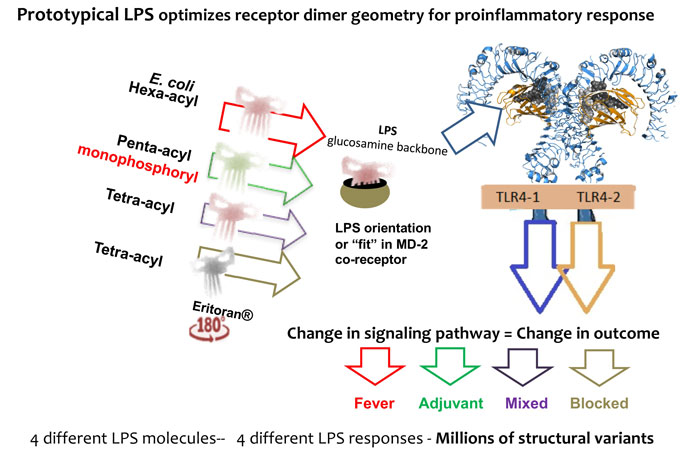

Figure 3: A change in LPS structure changes TLR4 dimerisation geometry, which changes the signalling pathway, changing the cytokine mix, which changes the event outcome based on a population of such events.
LPS structural change brings geometrical change to the receptor (MD-2/TLR4) and thus adapter recruitment and signalling change, see Figure 3.
Pattern recognition receptors (PRRs) were only theoretically proposed recently by Charles Janeway in 1989, and subsequently proven to exist in the mid-1990s. Since then, 10 toll-like receptors (TLRs) have been characterised in man and one of them, TLR4, was shown to be the LPS receptor. The TLR4 complex has two pathways that include a proinflammatory (MyD88 dependent) and an immunostimulative pathway (MyD88 independent) that has been associated with adjuvanticity.20
Hidden endotoxin
‘Hidden’ means non-detected by traditional assays because one cannot see non-prototypical or masked forms. Three mechanisms fall into the category of hidden endotoxin:
- Protein-bound
- Surfactant-masked (as in LER)
- From bioburden as low-dose or low-potent.
Given the commonality of fever responses to therapeutic proteins sometimes of unknown aetiology,21 combined with the tenacity of masking and the difficulty of thoroughly resolving masking mechanisms, the existence of ‘hidden endotoxin’ warrants further exploration.
Endotoxin and protein form complexes that are not detectable via Limulus-based methods. This will be reviewed by looking at a historical study by Petsch. In historical vaccine production, the propensity of the proteins to be contaminated with endotoxin is well documented.22 Some modern proteins routinely produced for analytical testing have also been shown to contain endotoxin at levels above the certificate of analysis specification.23
Protein-endotoxin binding
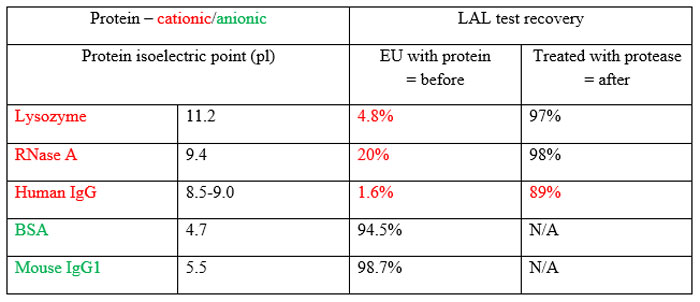

Table 1 Human IgG is the scaffold for monoclonal antibodies as therapeutic products26
Endotoxin-protein binding, particularly in cationic proteins, is a well-known issue and serves to hide endotoxin from detection by Limulus-based tests. Petsch et al 24 demonstrated that merely testing cationic proteins, even at low protein concentration (1mg/mL) and in pH-neutral solutions (=7) without some kind of pre-treatment, gives vastly understated results (see Table 1). Without pre-treatment using proteinase K, the endotoxin recovered was less than 2% of that added. Even after destructive treatment, a significant amount of the original endotoxin remained undetected. In another paper, Petsch and Anspach describe examples of the difficulty of removing endotoxin from proteins.25,26 While today’s protein removal methods include powerful tools, the development and performance of this set of procedures depends upon user proficiency as well as the difficulty level of the process.27
If one adds endotoxin spike to a mAb solution to demonstrate endotoxin reduction and subsequently removes it via chromatography, it may indeed remove all the endotoxin added. However, the initial solution can contain an initial ‘hidden’ amount that can only be detected by performing a pre-treatment. This is what the Petsch study showed. In such a case a Limulus-based test cannot be the final arbiter of content. Yes, the added endotoxin was removed in a reduction study, but this was perhaps because the binding sites were occupied by a pre-existing residual endotoxin. Proteases, as proteins, are also often contaminated with endotoxin! The Hyglos method has been shown to demask protein-endotoxin bound proteins as well as surfactant masked proteins.
Low endotoxin recovery (LER)
LER has been a controversial topic since its discovery by Chen and Vinther (Genentech) in 2013. The implication that low- or non-pyrogenic endotoxin could pose an issue in biologic products by stimulating the immune system has been a disconnect to industry participants used to having the bacterial endotoxin test (BET) serve a single purpose, ie, preventing fever in finished drugs. The fact that fever is common in biologics administration also seems widely disregarded. Nevertheless, biologics manufacturers have enacted a number of efforts to preclude artefacts that are immune stimulating. Verthelyi and Wang coined the term, innate immune response modulating impurities (IIRMIs), for the new relationship of artefacts with biologic products. Important strides have been made in gauging impurities (host-cell proteins, host-cell nucleic acids) as well as contaminants (MAMPs including flagellin and β-glucan28,29). The phenomenon is well established, but its full application to endotoxin is still resisted.
A study by Schwarz et al30 supports the knowledge of immune reactivity in the absence of Limulus-based activity. This is independent confirmation of FDA research on IIRMI (Verthalyi and Wang, and Haile et al). Researchers produced an r-protein using an E coli expression system and extracted it with a detergent, Triton X-100. Downstream, the residual detergent along with a chelating buffer served to mask the endotoxin, a natural form of a living expression system. They showed that the masked, Limulus non-reactive endotoxin reacted in a TLR test31 as well as a monocyte activation test. Using flow cytometry on the LER solutions, they also showed cell surface marker activation on monocytes (CD40, CD80, CD83 and CD86). Here, protein extraction upstream used a detergent (Triton-X) that brought masking downstream. Previously, masking had only been seen when polysorbate and a chelator occurred in downstream formulation constituents.
Bioburden: low-dose or low-potent endotoxin
Theory, disdained by some as ‘only theory’, dictates the tasks that constitute microbiological control. Since some bacterial types produce LPS of rather low reactivity32 with Limulus or rabbit tests, and since these are LPS types that vaccinologists are using to provide immune stimulation in the next generation of vaccines, perhaps they should not be reflexively dismissed. Water systems, as well as manufacturing process streams, are tightly controlled so as not to grow organisms. However, sometimes they do. If one sees Gram-negative bioburden and the absence of endotoxin activity, then the new paradigm may dictate further investigation. The practice of breaching bioburden limits or setting lenient limits is sometimes only realised after regulatory scrutiny.
The following are quotes from FDA biologics inspections33 and BioPhorum’s Operations Group34:
- “Multiple lots of drug substance were released, which had unacceptable high levels of bioburden during the final purification steps.”
- “Hold times for four column eluates [column steps x, y, z) have not been adequately validated microbiologically. No microbial limits are established for these processing steps, and results show high bioburden levels (>1,000 CFU/mL).”
- “…during the harvest of the unprocessed bulk, sublots of Lot xxxx and Lot yyyy exceeded the established action limits for bioburden.”
- BPOG: “A TNTC^35 result for any in-process bioburden sample should automatically result in an Action Level or Out Of Specification (OOS), which requires an investigation.”
Low-dose endotoxin has been found to stimulate immune systems.36 BET is currently based on calculations tied to the occurrence of fever (350EU per body dose). However, the FDA has for years been ratcheting down the permissible level of endotoxin in biologics. This can be seen in the 2012 Q&A Guideline37, where they recommend testing at the lowest practical, non-interfering level.
BIOGRAPHY
Kevin Williams worked for Eli Lilly for 30 years, developing QC tests for endotoxin detection, process control and control strategies of raw materials/excipients and container/closures. He wrote and edited the 2nd and 3rd Editions of the book, Endotoxins. He currently works for Hyglos-bioMerieux which has proprietary endotoxin detection methods including recombinant Factor C, a phage-based endotoxin capture technology, and comprehensive endotoxin service solutions with a special focus on low endotoxin recovery (LER).
References
- ‘Artefacts’ is a term used here for microbial MAMPs or PAMPs to include contaminants, impurities (from expression system) and mimetics (particles and aggregates that the body can confuse for infectious agents).
- Suvarna et al. Case Studies of Microbial Contamination in Biologic Product Manufacturing. Amer. Pharm. Review. 2011. Jan/Feb.
- Safety of Biologics Therapy. Brian Baldo. Springer. June 2016.
- Shaking from high fever.
- DellaGioia et A critical review of human endotoxin administration as an experimental paradigm of depression. Neurosci Biobehav Rev. 2010;34(1):130–143.
- fda.gov/safety/medwatch/default.htm
- CFR 314.80: “within 15 days of receipt … required to report to FDA …events classified as ‘unexpected’ or ‘unlabeled’ and ‘serious’.”
- Mimetics: aggregates, particulates and emulsions that the body can confuse for infectious structures.
- Kotarek et al. Subvisible Particle Content, Formulation, and Dose of an Erythropoietin Peptide Mimetic Product Are Associated With Severe Adverse Postmarketing Events. J Pharm Sci. 2016;105(3):1023-7.
- All of these microbial mimetics have been used as vaccine adjuvants.
- Bracewell et al. The future of host cell protein (HCP) identification during process development and manufacturing linked to a risk‐based management for their control, Biotechnol Bioeng. 2015;112(9):1727-1737.
- Briggs and Panfili. Quantitation of DNA and protein impurities in biopharmaceuticals. Chem.1991;6(9):850–859.
- In process development, polyclonal antibodies are formed against all proteins except the therapeutic protein to aid identification and preclusion.
- Huang et al. Use of Toll-Like Receptor Assays to Detect and Identify Microbial Contaminants in Biological Products. J. Clin. Microbiology. 2009.
- Darveau and Chilton. Naturally occurring low biological reactivity lipopolysaccharides as vaccine adjuvants. Expert Review of vaccines. 2013;12(7):707-709.
- There have been dozens of endotoxin detoxification methods identified in the search for vaccine adjuvants.
- Vertheyli and Wang. Trace levels of innate immune response modulating impurities (IIRMIs) synergise to break tolerance to therapeutic proteins. PLOS ONE. 2010.
- Haile et al. Detection of IIRMIs in therapeutic proteins. PLOS ONE. 2015.
- 2014 FDA Guideline: Immunogenicity Assessment for Therapeutic Protein Products, see Section 5 page 18, impurities with adjuvant activity.
- Wang et al. Kdo2-Lipid A: Structural diversity and impact on immunopharmacology. Rev. 2015.
- Baldo: “The mechanisms of mAb-induced infusion reactions are not yet understood.”
- Brito and Singh. Acceptable levels of endotoxin in vaccine formulations during preclinical research. J Pharm Sci. 2011;100(1):34-7.
- Schwarz, Schmittner, Duschl and Horejs-Hoeck. Residual Endotoxin Contaminations in Recombinant Proteins Are Sufficient to Activate Human CD1c+Dendritic Cells. PLOS ONE. 9(12):e113840.
- Petsch D, Deckwer WD, Anspach FB. Proteinase K digestion of proteins improves detection of bacterial endotoxins by the Limulus amebocyte lysate assay: application for endotoxin removal from cationic proteins. Anal Biochem. 1998;259(1):42-7.
- Petsch and Anspach. Endotoxin removal from protein solutions. Journal of Biotechnology. 2000;76:97-119.
- “Today many cases are known – although not published – in which a pharmaceutically promising new substance was regarded pyrogenic while it turned out later that the origin of pyrogenicity was not the substance but endotoxins.”
- Saraswat et al. Preparative Purification of Recombinant Proteins: Current Status and Future Trends. BioMed Research Intl. 2013.
- Gefroh et al. Multipronged approach to managing beta-glucan contaminants in the downstream process: control of raw materials and filtration with charge-modified nylon 6,6 membrane filters. Biotechnol Prog. 2013;29(3):672-80. doi:10.1002/btpr.1718. Epub 2013 Apr 18.
- Vigor et al. Development of downstream processing to minimize beta-glucan impurities in GMP-manufactured therapeutic antibodies. Biotechnol Prog. 2016;32(6):1494-1502. doi:10.1002/btpr.2359. Epub 2016 Oct 21.
- Schwarz et al. Biological activity of masked endotoxin. Nature Sci Rep. 2017;7:44750.
- A cell-based TLR4-NF-κB-luciferase reporter gene assay.
- Darveau and Chilton. Naturally occurring low biological reactivity lipopolysaccharides as vaccine adjuvants. Expert Review of vaccines. 2013;12(7): 707-709.
- Lolas et al. CMC Microbiology Review of Biologics License Applications and Pre-License/Pre-Approval Inspections: Therapeutic Biological Proteins. American Pharmaceutical Review (on-line). 1 March 2010.
- Bain D. Microbial Monitoring For Biological Drug Substance Manufacturing: An Industry Perspective. BPOG, May/June 2015.
- TNTC is “too numerous to count”, not a very strict criteria for OOS.
- Chen et al. Super-low Dose Endotoxin Pre-conditioning Exacerbates Sepsis Mortality. 2015;2(4):324–333.
- fda.gov/downloads/drugs/guidances/ucm310098.pdf
- “Cells of the innate immune system, in effect, give permission to the cells of the adaptive immune system to respond to antigen.” Roitt’s Essential Immunology. Delves et al. 13th Wiley. 2017, pg 221.




