Managing contamination risks in glove holes in barrier separation technology
Posted: 20 October 2016 | Corinna Maier, James L. Drinkwater | 6 comments
The manufacture of sterile medicinal and therapeutic products increasingly calls for aseptic processing using barrier separation technology comprising isolators and restricted access barrier systems (RABS) that have glove-sleeve systems.

Such glove-sleeve systems are sealed to the barrier screens via glove ports. As vulnerable polymer membranes, the glove sleeves are the weakest link in the barrier’s integrity and present a risk of microbiological and particulate contamination transfer1 into the barrier enclosure as a result of glove holes that may not be detected by visual inspection. In addition, there have recently been regulatory observations of over-reliance on visual inspection in RABS barrier gloves during glove management strategies.
This article considers a glove management strategy, furthering the understanding of the factors that put European Union Grade A controlled environments at risk from microbiological contamination. To manage risks in process operations, a strategy of periodically replacing glove sleeves (before failure) and testing for leak integrity before and after glove use is necessary.
Overview of challenges
Barrier glove-sleeve systems extend/intrude into Grade A process zones of isolators and RABS (see Figure 1), during process operations for the following:
- Environmental monitoring plate placement/recovery as inherent interventions
- Process set-up before and after vapourised hydrogen peroxide bio-decontamination
- Inherent process interventions, for example, charging stoppers in feeder bowls
- Corrective interventions, for example, recovery from a fallen, broken or jammed product container.
Although operators should be complying with ‘first air’ principles with good aseptic technique; i.e., not positioning gloved hands over critical surfaces or contacting critical process points so that the first air that leaves the down-flow HEPA filter can reach critical surfaces in an uninterrupted fashion, the close proximity of gloves to critical process points increases risk of contamination if the gloves have holes. Isolator and RABS systems often occur in combination to form controlled environments for pharmaceutical product filling (see Figure 2), with each gloved manipulation presenting different risk levels.


Figure 1: Isolators and RABS barrier gloves
Increasing knowledge of risks with glove ‘pin holes’
Previous studies on the risks of contamination transfer through pin holes in barrier isolator technology gloves have contributed to the understanding of the risks relative to realistic bioburden challenges in surface-to-surface contamination transfer and airborne contamination as a result of handling contaminated test surfaces2 . In addition, considerations have been provided on the necessary holistic approach to glove hole risk management3. The conclusions from previous studies4 underestimated the full extent of risks owing to the fact that not all potential contamination transfer routes were considered.
Further studies5 indicate that another operational risk at glove entry involves a momentary pressurisation effect generating a jet stream of background environment grade air into the Grade A process environment. However, the risk of this potentially contaminating event is transient and once a hand is in position, the risk reverts back to previously reported (lower) levels.
Contamination transfer routes from glove holes
There are three glove operational states that pose a risk of contamination transfer via glove holes (described below) and consequently knowledge of risk management-related glove management rationale is required. In addition to potential routes of contamination transfer, the glove hole size has a significant impact on contamination risk and must be considered when defining response to detected holes.
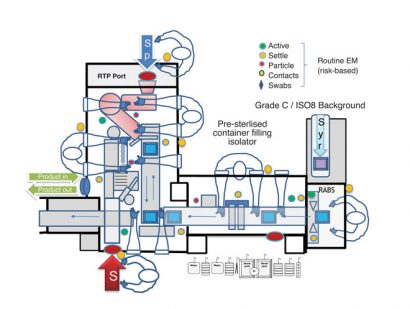

Figure 2: Operator Glove access into Isolator Barrier system
Holes in gloves not in use
Isolator manufacturers have completed studies to confirm the potential for contamination transfer when gloves are fitted to a positive pressure isolator but are not in use. Contamination transfer studies were completed at 100 micron glove hole sizes; the limit of detection of most commercially available glove-sleeve leak integrity test devices. Results, as expected, indicate the positive pressure air out-flow from glove hole leaks do not translate into contamination transfer into the isolator Grade A critical zone (in effect, a reverse direction to airflow).
Surface contact contamination
Studies of potential surface-to-surface contamination transfer are significantly impacted by glove hole size. Glove holes in the range of glove tester detection (100 micron) present a capillary path for microbiological physical transfer and, together with positive pressure differentials from the isolator Grade A environment to the surround, have demonstrated a low risk of contamination transfer – particularly at the low bioburden levels typically found in pharmaceutical cleanrooms6 . However, the size of the glove hole does escalate contamination transfer risks and hole sizes above the typical visual inspection level of reliable detection (400-500 micron) start to result in contamination risks.


Figure 3: Jet streams of contaminated air at glove hand entry
Glove entry into isolator and RABS
Studies indicate that gloves can develop momentary pressurisations of up to 500 Pa as operators’ hands pass the more restricted wrist section, or at entry into finger pockets. Once in position, however, pressures normalise to the pressure differential as a function set for the barrier system. This momentary pressurisation creates a jet stream of high velocity incoming air, which comes from the surrounding environment so potentially carries both airborne particulate and microbial contamination (see Figure 3).
Computational fluid dynamics (Figure 4) have been used to characterise the jet streams of contamination within a Grade A isolator environment with down-flow air at a flow rate of 0.45m/s. For example, it was previously found that in a 100 micron-hole jet stream with 500 Pa pressure in a glove, the jet stream was carried 90mm before the velocity dropped to the extent that the down-flow air became more dominant; i.e., the cross flow stream was re-directed downwards. Studies of the jet stream were also completed as a simulation of the glove entry transition where pressure rises momentarily (see Figure 5).
Glove management strategy
With awareness of the potential contamination transfer routes through glove holes such as these, risks can be managed with a manage ment strategy comprising three parts:
- Periodic glove replacement
- Glove leak integrity testing and methods: considerations for visual and physical testing
- Response to detected glove holes.


Periodic glove replacement should be based on glove performance without degradation that may compromise leak integrity with consideration of stress factors7 that are applied in routine use over the life cycle of the glove. These may include: integrity testing (by pressurisation); hydrogen peroxide vapour exposure; physical manipulation in process operations; and re-integrity testing.
As glove leak integrity testing is required within controlled rooms/operational areas, ‘clean’ methods are required that do not themselves present a risk of contamination to the environment or process/product. Such methods are typically based on localised pressurisation of the glove sleeve, achieved by sealing the glove port with a test disc. Testing devices that have control limits and clear acceptance criteria – in addition to compiling leak integrity reports that can be retained as records – represent adherence to good manufacturing practice.
The frequency of leak integrity testing will depend on the application, but in principle all gloves on a barrier separation system should be integrity-tested before use, followed by testing post-use. For long campaigns where the isolator and/or RABS system is operated with an aseptic hold (between system decontamination steps), a different approach to pressurising the glove into the Grade A process zone would be required. Glove test discs that can be held in the Grade A zone and facilitate test pressurisation from Grade A to the surround (out-leakage) are now available for campaigns of leak integrity testing.
Glove leak integrity testing should comprise, firstly, a physical test using an integrity test device with a limit of detection at least in the 100 micron range and test pressure above 800 Pa; and secondly, visual inspection by trained operators. Overreliance on visual inspection that, at best, detects 400-500 micron-hole sizes can be considered unacceptably risky. There have been cases of deficient practices reported whereby RABS gloves were only tested physically with a very basic ‘water leak test’ (i.e., visually inspecting a water-filled glove for leaks).
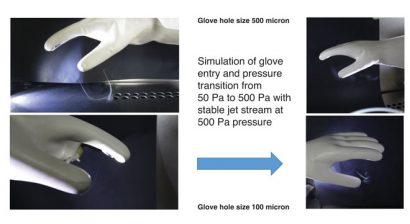

Figure 5: Jet streams of contamination through transitions pressure of 50 Pa to 500 Pa
A strategy is also required for responding to the detection of glove leak ‘pin holes’8 . Such a strategy should consider the location of the failed glove and proximity to critical contamination risk points and, hence, the extent of impact and contamination risk. Additionally, results should be trended and periodically reviewed to see if there are any repeated location glove failures, suggesting the glove is at an unnecessary risk of damage.
There are different contamination risks between barrier gloves on RABS and isolators owing to the different levels of contamination challenges resulting from varying surrounding environment classifications, e.g., for filling EU Grade C for isolators and Grade B for RABS and higher levels of operator gowning for RABS/ Grade B zones.


Overreliance on visual inspection is unacceptably risky
Gloves are bio-decontaminated/sterilised in different ways depending on whether they are intended for RABS or isolator use. The specific testing strategies are as follows:
Procedure for RABS
All RABS gloves need to be physically tested before moist heat (autoclave) sterilisation. This could be carried out at a ‘test station’ that is off-line to the RABS and in a separate area. Where a sterilisation process is not used and only a sanitisation step employed, a rationale and risk assessment would be required. In addition, there needs to be qualification that the sterilisation process and aseptic assembly of RABS gloves in-to-place does not compromise the glove leak integrity. Prior to use (in-place) RABS gloves need to be visually inspected and one should physically test, either on-line in the RABS barrier or off-line at the test station, the gloves that have been used in process operations. All results should be recorded and trended and a periodic review of the data to assess any trends in glove failures as a result of leak hole detection completed. Any detected glove hole leaks need to be investigated, particularly after use, and impact and risk assessments related to risks of contaminating the Grade A process environment – and potentially sterile product – completed. Further data from environmental monitoring and sterility tests should also be reviewed when considering the potential impact on product contamination. Finally, in-place physical glove leak integrity testing during extended campaigns and aseptic holds should be considered.
Procedure for isolators
All isolator gloves in place on the isolator barrier should be physically tested, which typically takes place within an automated hydrogen peroxide vapour decontamination cycle, with visual inspections occurring after glove use and during production operations. There should be complete physical testing of gloves used in production operations at the end of batch processing, and any glove holes detected should be investigated, with an impact and risk assessment related to contamination risks conducted. In addition, one should consider the campaign’s physical glove leak integrity testing.

RABS and isolators call for differing contamination risk management strategies
Summary
The more risk-based initiatives – such as quality risk management – apply, the more we have to consider glove management and potential contamination risks from glove ‘pin holes’ holistically. Understanding that there are also potential contamination transfer risks from glove holes (as a result of momentary pressurisation at glove entry) increases the need to re-assess thinking on the levels of risks and risk management in glove manipulation operations. The jet stream leak path is momentary and short, so provided gloves are entered close to the barrier wall, near return air ducts and at low level, such risks can be mitigated.
There are different levels of contamination risk and varying procedural methods of integrity testing between RABS and isolators that need to be addressed in a glove management strategy. For best practice such a strategy is based on an holistic approach considering risk knowledge of contamination routes, glove hole detection as an in-process control, glove replacement before degradation, ways of to reducing the risk of failure and actions to be taken in the event of hole detection.
About the authors


As Deputy Head of aseptic processing technologies and GMP compliance at Franz Ziel GmbH, Corinna Maier is involved in barrier technology projects, isolators, RABS, material transfer chambers in applications of production, and clinical trial scale filling and sterility/product testing. Developing environmental monitoring programmes is another scope of her work. In addition, Corinna is involved in normative work at ISO with the revision of ISO 13408-6 for isolator systems. Corinna studied at the University of applied sciences AlbstadtSigmaringen specialising in hygiene technology. Following her Bachelor thesis she started working at Franz Ziel GmbH in 2014. This final exam included an assessment of current technologies for physical glove testing and the impact of small-sized glove holes in different applications. Her current specialisation is gaseous disinfection using hydrogen peroxide vapour (vH2O2) technologies.












Hi,
Very Useful Article.
Hypalon gloves fixed to oRAB are being exposed to adjacent Grade B environment when the doors are opened for interventions. How to protect them being contaminated when exposed to Grade B conditions. Though these gloves are sanitized with 70% IPA before the doors are closed, it is expected to get contaminated. Please advise. Any help of extending the LAFs to protect the open doors?
Thanks.
Ben
Hi,
Thanks for the article!
I would like to know how often do I have to perfom the leak test? before and after each sterility test?
Hi. Can you share again the link where we can find the references? The shared one isn’t working anymore.
Thanks
Hello,
The link for the reference shared in the comment below has been updated. Here is the link also: https://www.europeanpharmaceuticalreview.com/article/44775/issue-5-2016-digital-edition/
Very helpful article, however, I did not see how to obtain the references that were cited
Hi,
Please find the link for the issue PDF <a href="https://www.europeanpharmaceuticalreview.com/article/44775/issue-5-2016-digital-edition/“>here.
The article is on pages 48-52, with the references on page 52.