Ten steps to success
Posted: 20 May 2005 | | No comments yet
The technology transfer management process reflects an integrated approach to the best practices benchmarked.
A well-defined and well-designed technology transfer process is critical for ensuring successful commercial launch. Technology transfer includes manufacturing, packaging and test method transfer to commercial sites.
The technology transfer management process reflects an integrated approach to the best practices benchmarked.A well-defined and well-designed technology transfer process is critical for ensuring successful commercial launch. Technology transfer includes manufacturing, packaging and test method transfer to commercial sites.
The technology transfer management process reflects an integrated approach to the best practices benchmarked.
A well-defined and well-designed technology transfer process is critical for ensuring successful commercial launch. Technology transfer includes manufacturing, packaging and test method transfer to commercial sites.
The strategy for all technology transfer activities needs to support the goals of implementing the business process. Specifically, the success of the strategy should manifest itself in time through the following benefits:
- Supports robust filings and expedited regulatory approval
- Critical to global launch readiness
- Essential to ongoing manufacturing site success
Transfer Team mission
The Technology Transfer (TT) Teams referred to in this process are cross-functional, integrated teams that are ultimately responsible for managing the successful strategic planning and execution of technology transfer projects.
TT Teams are empowered by functions to make decisions and should be represented by the same functional member throughout the life of the transfer project. TT Teams navigate a project through a Milestone Review Process and manage formal expectations with the Technology Transfer Review Board via the use of project contracts
To ensure the successful transfer of products and processes, TT Teams will:
- Coordinate process scale up, site transfer, validation and PAI readiness activities
- Assure strategic coordination and integration across drug substance, drug product, analytical methods and facilities development work streams to optimise process, logistics and cost
- Manage an integrated project plan and contract
- Provide technical expertise and support to receiving sites post-validation until the TT Team is formally decommissioned
- Assure that TT risks are managed and suitable risk mitigation strategies are in plac
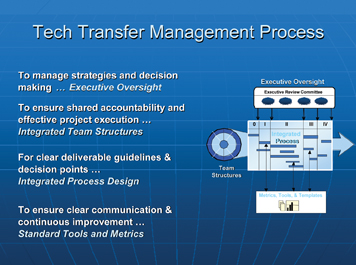
To achieve those goals mentioned above, as well as successful completion of the various tasks, the technology transfer team follows a well-structured management process (Figure 1):
- Executive Oversight: a body of senior executives representing most of the operational areas within a pharmaceutical organisation. Provides governance that guides, coaches and holds integrated technology transfer teams accountable and responsible for the timely and successful completion of projects.
- Integrated Team Structure: a team consisting of 12 to 16 individuals, representing the main stakeholders of the project.
- Integrated Process Design: a clearly written and defined project plan with agreed due dates and deliverables.
- Standard Tools and Metrics: Metrics to assess the success and performance of the team (i.e. attendance, adherence to pre-established timeline measuring the extent of deviations to this timeline (weeks, months).
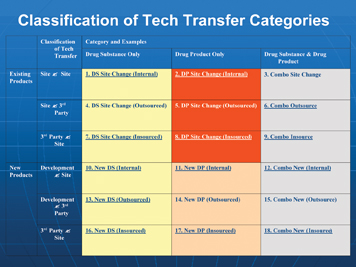
Classification of technology transfers
Figure 2 demonstrates how many different technology transfers could be conceivably possible in an organisation.
However, the integrated technology transfer, as a comprehensive business process, is applicable to any of these potential technology transfers.
The ten steps to a successful technology transfer
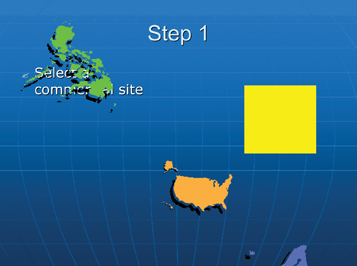
Step 1: Site selection
The site selection process may be company-specific, depending on which manufacturing philosophy an organisation is following i.e.: centres of excellence by dosage form, or several large, multi-functional manufacturing centres strategically located across the globe (Figure 3).
However, there are some general points to consider:
- Location
- Ethnic, cultural diversity
- Capacity versus utilisation
- Technology versus process design
- Tax implications
- Import/export laws
- Shipping conditions and costs
- Safety, industrial hygiene
- Regulatory
- Capital and expenses
Listed below are some challenges that should be examined early on in the process, in order not to trip over them at a later stage:
Shipping conditions and costs: depending on the nature of the product and active substance, it might pose a significant challenge to get the active substance to the manufacturing location and the finished pharmaceutical bulk product (especially sterile formulations) or finished packaged product back to the distribution centres (i.e. cold chain shipping in and out of tropical regions).
Capital and Expenses: determine a project budget early on, taking into consideration travel and accommodation expenses, replacement or spare parts required for the new product (dedicated exchange parts) and also ensure that the facility and equipment is operational, qualified and meets the operating ranges for the new process. It can become very costly to qualify major pieces of equipment in a rush via contract resources and may lead to unanticipated delays in the project.
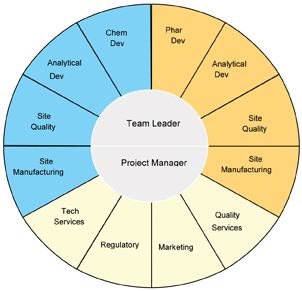
Step 2: Form the technology transfer team
The integrated technology transfer team manages all aspects of a technology transfer. The team leader has primary responsibility for project leadership. The project manager is responsible for the planning, timeline management and facilitation of team meetings and team members’ activities and drives the adherence to processes and standards.
The integrated team members (Figure 4) assure strategic coordination and integration between drug product and drug substance technology transfers (including all analytical aspects); they identify and manage process dependencies, timing dependencies, assure that risks are managed and mitigated and manage resource needs. The core team members are also responsible for managing associated sub-teams from the respective area they represent (i.e. analytical method development for drug substance, drug product and microbiological limits), ensure timely execution of finite tasks and are the conduit between the core team and the executing sub teams.
It is imperative to the success of the project that the technical team leads and that the project manager establishes and maintains excellent channels of communication between each other and the rest of the team. To sustain an efficient working group it is also imperative that the lead and project manager seek conflict resolutions off line before a general team meeting, through mediation and active development of alternate solutions.
Step 3: Plan the project
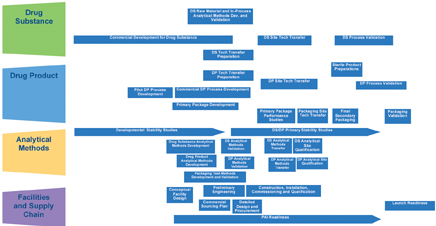
The technology transfer integrated process design provides guidance across major work streams (Figure 5).
The purpose of this high-level project plan flow chart is to visualise the activities across the four major components that constitute a technology transfer and help identify potential risks and challenges.
The next step of finite planning will be to develop process work flow charts and outline key deliverables for each major activity identified in the various boxes outlined in the integrated process design.
Step 4: Perform gap analysis
The gap analysis is one of the first major activities that will follow the design of the integrated flow chart. Each area will identify their needs and wants, as required to meet their committed due dates and deliverables.
A well established guide to assess the gaps are detailed process flow charts, indicating the equipment and batch size required for the production of the product. Gap analysis should also focus on auxiliary requirements such as special consideration for protective equipment, area designs (humidity controls, safety, explosion proof manufacturing suites and equipment, solvent and waste stream handling, water quality). This information is crucial for planning an accurate overall project plan. One should not underestimate the effort required to upgrade existing utilities, facilities and equipment, to expand existing capacity or to install and qualify newly acquired resources.
Step 5: Create and update the strategy
The technology transfer core document consists of the following elements:
- Project scope
- Technology Transfer strategy
- Key issues and challenges
- Project background
- Product overview
- Characteristics and formulation
- Drug substance process
- Drug product process
- Analytical methods
- Facilities
- Specifications and batch strategy
- Project plan
- Key assumptions
- Critical milestones
- Resources – team membership
- Capital requirements
- Overall milestone plan
- Risk management and mitigation
Two key sections of this document are:
a) specifications and batch strategy. It is recommended to establish and agree up front to a detailed design of experiments (DOE) that will be used to define or confirm in process specifications, critical processing parameters and critical quality attributes of all stages of the process and finished product. The next step in the batch strategy is to assess how many batches are required to establish operator proficiency before engaging into the manufacture of site stability and process validation batches. The entire strategy requires a careful balance between API requirements and API availability as well as production schedule and project timeline.
b) Risk management and mitigation: a thorough analysis of what might go wrong or experience potential delays during the project execution is helpful in avoiding bad surprises and a scramble for hasty solutions. Focus should be on outlining realistic mitigation plans that have the least impact on the overall project flow and project finances.
Step 6: Execute the strategy
During the execution of the technology transfer, the team should consider and pay special attention to the following points:
- Track the plan
- Monitor milestones
- Anticipate slippage early
- Site readiness
- Suppliers
- Raw material inventories
- Monitor risks
- Manage the financials (consider approval timelines for capital requests)
It is strongly recommended to assign a team member to monitor and track the spending of capital funds and ensure that the project stays within approved limits.
Step 7: Manufacture technology transfer batches
A pre-requisite to beginning the manufacture of technology transfer batches is a well thought out and established DOE that will produce expected results, which are statistically defendable.
The purpose of technology transfer batches is also to define (or confirm from earlier development batches) the critical process parameters, critical quality attributes and critical in-process tests. It is also recommended at this point to challenge process parameter boundaries (i.e. wet/dry conditions).
It is also a good opportunity to confirm or establish equipment capabilities i.e. compression speeds, packaging line capabilities etc. This information is very useful for the planning department to establish preliminary labour standards, as well as estimate the time required to produce initial launch quantities.
Step 8: Manufacture process assessment batches and validation batches
At this point, the process should be defined and process limits well understood. It is an opportunity for the receiving site to review and finalise the written manufacturing directions, challenge the validation sampling plan and review and improve material flow and material handling procedures, challenge operator proficiency and process understanding as well as adherence to newly created, product specific SOPs. Once a confidence level has been established that facility, equipment, process, analytical test methods and operator understanding reached a reproducible and reliable level, the site is ready to execute the manufacture of validation batches.
Step 9: PAI readiness
Establishing PAI readiness is not an activity at the end of the project (= scrambling to assemble and collect pertinent documents and reports). Establishing PAI readiness is an on going activity for the duration of the technology transfer project and maintaining a running log of documents and reports as they are being prepared will help to achieve PAI readiness at the end of the project almost instantaneously.
Step 10: Celebrate achievements and project success
Once all the project milestones have been successfully completed on time and on the basis that a regulatory inspection has not uncovered major loopholes or deficiencies in the site’s production readiness, it is time to recognise the achievement of the project team.
To foster cross-functional thinking, break down intellectual and departmental silos, it is important to recognise the team as a whole and not individuals. However, that philosophy depends on the company’s management style and how they value their colleagues.
Transferring pharmaceutical technology is not brain surgery or rocket science. Transferring pharmaceutical technology is simply using common sense management tools, which are:
- Communicate
- Value each other’s opinion and input
- Develop a genuine understanding of every area’s predicaments and challenges
- Resolve issues together
- Communicate more