Examining receptor activation: New technologies reveal how G protein-coupled receptors recognise ligands and talk to intracellular partners
Posted: 24 June 2010 |
Heptahelical G protein-coupled receptors (GPCRs) are arguably the most important single class of pharmaceutical drug targets in the human genome. According to Overington, of the 266 human targets for approved drugs, a remarkable 27 per cent correspond to rhodopsin-like, or Family A, GPCRs. Despite recent dramatic advances in targeting of kinases, the continued success of monoclonal antibody-based therapeutics and the advent of new drug entities like siRNAs, GPCRs remain the pre-eminent class of drug targets…
With perhaps 100 receptors still categorised as orphan (i.e., tissue-specific receptor expression can be documented but no endogenous ligand has been identified) out of a total of about 726 GPCR genes in the human genome, it is likely that GPCRs will continue to be robust drug targets in the foreseeable future. The discovery of small-molecule allosteric GPCR ligands, so-called biased ligands, and ‘pepducins’ also might allow targeting of ‘refractory’ GPCRs that resist conventional drug development strategies. Receptor oligomerisation, or in some cases hetero-oligomerisation, is thought to modulate receptor cell-surface expression, ligand-binding affinity, and downstream signalling specificity for at least some classes of GPCRs and targeting receptor oligomers might also prove possible.
Recent reports of crystal structures of native and engineered GPCRs such as rhodopsin, opsin, A2A adenosine, β2- and β1-adrenergic receptors provide insights into the molecular mechanism of GPCR activation. With the exception of the opsin crystal structures, the rhodopsin, β1AR, β2AR and A2A structures represent the inactive conformation, which are stabilised by inverse agonist or antagonist binding. In contrast, the structure of opsin (the apo form of rhodopsin, which is the archetypical GPCR of visual phototransduction) bound to a 11-residue-long peptide representing a variant of the C-terminus of the alpha subunit of transducin (the cognate G protein of rhodopsin) may provide clues to an activestate receptor.
However, a wealth of pharmacological and biochemical data suggest that probably for most GPCRs, a fully-active state of the receptor might only exist when it is in a ternary complex with an agonist ligand and a heterotrimeric G protein. The key question can be formulated as follows: “How does the binding of an agonist ligand, generally originating on the outside surface of the cell membrane receptor, cause the release of bound guanosine diphosphate (GDP) on the alpha subunit of a heterotrimeric G protein located on the inside of the cell at a distance of perhaps seven to 10 nanometres?”
In the absence of a high-resolution structure of at least a representative of such a ligandreceptor- G-protein ternary complex, the mechanism of receptor-mediated G-protein activation remains formally unresolved. However, a number of technologies are beginning to provide a new set of tools to address with chemical precision how ligands activate receptors and how receptors talk to cellular G proteins.
My laboratory is interested in uncovering the principles that underlie ligand recognition in GPCRs and understanding, with chemical precision, how receptors change conformation in the membrane bilayer and activate G proteins when ligands bind. Using vertebrate vision as a model system, we have developed an interdisciplinary approach that employs a number of new converging technologies:
1) All atom and coarse grain molecular dynamics (MD) computer simulations of GPCRs in membrane bilayers in concert with experimental validation
2) Unnatural amino acid mutagenesis of GPCRs using amber codon suppression technology
3) Advanced FTIR (Fourier-transform infrared spectroscopy) and solid-state NMR methods to interrogate receptor dynamics
4) Nanoscale apolipoprotein bound bilayers (NABBs) as membrane mimics support structures for GPCRs
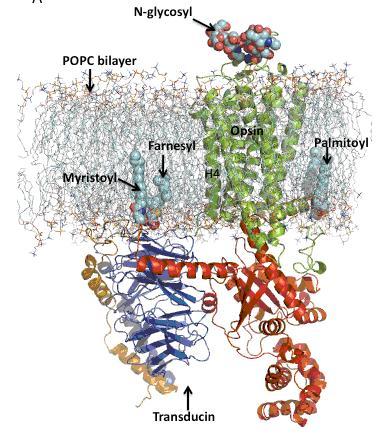

Figure 1 MD simulation model of the opsin-transducin complex in a POPC lipid bilayer. Opsin and transducin are drawn in ribbon format and post-translational modification on opsin and transducin are represented in space-fill style. Opsin is oriented in the bilayer such that helix 4 (H4) is perpendicular to the plane of the bilayer. The extracellular surface is oriented toward the top of the figure.
Computational approaches
Comparative or homology protein structure modelling is now a well-established method for building three-dimensional (3D) models of proteins of unknown structures (target) based on one or more proteins of known structure (template). The quality of the model increases with the sequence similarity and availability of a correct alignment between template and target. Both of these conditions are generally satisfied in building a 3D model of a protein complex using components with known crystal structures. Thus, comparative-modelling techniques can be adapted to predict a 3D structure of a protein complex using structures of the components that constitute the macromolecular assembly. For example, a putative functional receptor-Gprotein complex can be modelled using existing high-resolution structures of the constituent receptor and G protein.
In 2008, Scheerer et al. reported the crystal structure of the complex of opsin and a peptide variant of the C-terminus of alpha-subunit of transducin. The bound peptide provided some clues as to how the entire G protein might interact with the receptor. Superposition of the transducin (Gtαβγ•GDP) and opsin-peptide crystal structures, however, resulted in a severe steric clash between Gtαβγ•GDP and lipid bilayer. The authors suggested that the opsin-peptide structure might represent the opsin-transducin complex in which GDP has just left the binding pocket. Based on this assumption, they proposed a conceptual model of signal transmission from the active-state receptor to the G protein through a massive change in alpha5 helix packing in the alphasubunit of transducin by a 40° tilt of the helix away from the remainder of alpha-beta-gamma subunits of transducin. We used a slightly different approach to model the complex of an active-state opsin with the heterotrimeric G protein, transducin (Gtαβγ•GDP).
We modeled the entire opsin–Gtαβγ•GDP complex by building it up from bits and pieces using available highresolution structures. Using the NMR structure of an 11-residues peptide from the C-terminus of the alpha-subunit of transducin (GtαCT; PDB access code: 1AQG) and the structures of opsin in its G-protein interacting conformation (PDB access code: 3DQB), of rhodopsin in an inactive state (PDB access code: 1U19), and of the heterotrimeric G-protein, transducin (Gtαβγ•GDP; PDB access code: 1GOT), we built a plausible model and then performed an all-atom (~150,000) MD computer simulation of the opsin–Gtαβγ•GDP complex in a palmitoyloleoyl- phosphatidylcholine (POPC) bilayer in a salt solution with physiological ionic strength to equilibrate the system. We also included all post-translational modifications of both the receptor and G protein. Preliminary analysis shows that our new alternate model of the opsin–Gtαβγ•GDP complex does not require massive changes in alpha5 helix packing for receptor-mediated G protein activation. The opsin-GtαCT complex model also differs from the crystal structure of opsin-GtαCTK341L complex in other ways. For example, whereas the GtαCTK341L peptide in the receptor-binding pocket has no side chain-side chain interactions with the receptor and is oriented towards helix 5 and helix 6 in opsin, in our molecular model of the opsin-GtαCT complex, GtαCT orients towards the solvent exposed cleft between helix 6 and helix 8 in opsin.
Figure 1 shows a snapshot derived from the MD simulations of the opsin-Gtαβγ•GDP complex in the POPC bilayer. Opsin is oriented in the bilayer with helix 4 perpendicular to the plane of the bilayer. The palmitoyl modifications on Cys322 and Cy323 in helix 8 (H8) in opsin anchor the receptor to the lipid bilayer with H8 positioned inside the lipid bilayer interface. In contrast, the N-terminus of alpha-subunit (GtαNT) and the C-terminus of gamma-subunits (GtγCT) of transducin are positioned outside the lipid bilayer interface with myristoyl and farnesyl groups on the alpha- and gammasubunits of transducin inside the bilayer. GtαNT lies parallel to the plane of the bilayer interface with positively charge surface of the amphipathic helix interacting with the negatively charged bilayer interface.
Our preliminary molecular model of the opsin–Gtαβγ•GDP complex provides structural insights into the role of cytoplasmic loops in the receptor in G protein recognition and activation. The model of the complex is also amenable to long-time scale MD simulations that we hope will provide the structural basis for under – standing receptor-mediated G protein activation. For example, the opsin–Gtαβγ•GDP complex model reveals several contact sites between the opsin and the alpha-subunit Gtα.. of transducin. These types of interactions, which are predicted by the model, can be used to design cross-linking experiments for bio – chemical validation using the site-directed unnatural amino acid mutagenesis technology developed in parallel.
Probing receptor activation using genetically-encoded unnatural amino acids
Unnatural amino acid mutagenesis is based on the principle of amber codon suppression. If an amber codon (UAG) is suppressed, read-through occurs during mRNA translation. Protein synthesis will be terminated at the next incidental nonsense codon to create a fusion protein with the native N-terminal portion and a fusion at its C-terminal end that represents a translation of 3’- mRNA sequence between the original amber codon and the next functional nonsense codon. Amber codon suppression requires a so-called suppressor tRNA and a corresponding amino acyl-tRNA synthetase, which charges the tRNA with an activated amino acid.
Because the amber codon (UAG) is used rarely in most cells, engineering on-demand amber codon suppression is a reasonable strategy. If the amber codon were used at high frequency, then any strategy to suppress amber codon termination would result in large numbers of read-through fusion proteins that might lose function and cause cell toxicity.
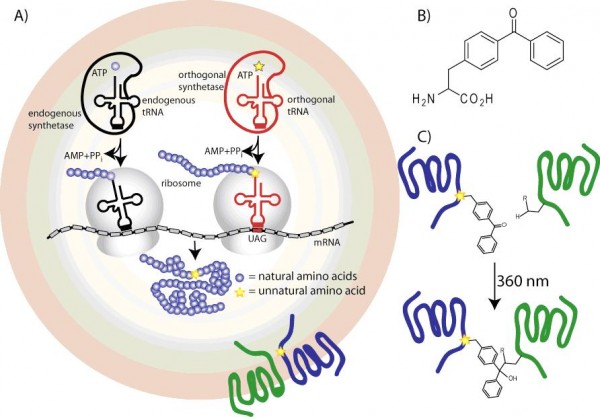

Figure 2 Scheme to encode genetically p-benzoyl-L-Phe into expressed GPCRs. (A) At the amber codon UAG, an engineered suppressor tRNA and orthogonal amino-acyl tRNA synthetase can incorporate an unnatural amino acid. (B) Structure of p-benzoyl-L-phenylalanine (pBpa). (C) In a photo crosslinking approach, site-directed unnatural amino acid mutagenesis is used to genetically encode pBpa into the blue receptor, which is hypothesised to interact with the green receptor. Upon irradiation with 360 nm light, pBpa will form a covalent bond to a side chain of the green receptor.
When a suppressor tRNA/amino acyl-tRNA synthetase pair is orthogonal, they form an exclusive and specific pair and will always introduce a discrete amino acid at the amber codon. The efficiency of amber codon suppression depends upon a number of factors, but can be easily measured. Now suppose that the amino acyl-tRNA synthetase can be engineered to charge its orthogonal suppressor tRNA, not with one of the 20 naturally occurring amino acids, but with an unnatural amino acid with an interesting chemical property. If the engineered orthogonal suppressor tRNA/amino acyl tRNA synthetase pair was introduced in the presence of the proper unnatural amino acid, then that amino acid would be incorporated into the growing peptide chain during protein synthesis at an amber codon. Furthermore, if a particular gene of interest were mutated to contain an amber codon, then the specific unnatural amino acid corresponding to the orthogonal tRNA/synthetase pair would be introduced at the site of the mutation.
A particular unnatural amino acid might contain a reactive chemical group or a spectroscopic probe. Every full-length expressed protein should contain the unnatural amino acid. Amber codon suppression might then be used to label a heterologously-expressed protein of interest at a specific location. For example, a p-azido-L-phenylalanine is a particularly useful unnatural amino acid. The azido group is a useful chemical tag, its vibrational signature can be detected in infrared spectroscopy experiments and it can be used in photochemical crosslinking studies.
Peter G. Schultz and others have refined and exploited the amber codon suppression technology for use in E. coli protein expression systems. However, GPCRs do not generally express in E. coli. The main technical challenge to using the technology in mammalian cells is to find and express the proper suppressor tRNA.
We recently developed a highly-efficient amber suppression system for use in mammalian cells in culture. We used a luciferase reporter system to optimise successively the expression of an effective suppressor tRNA and then a high-fidelity orthogonal amino acyl-tRNA synthetase. The amino acyl-tRNA synthetase genes were engineered to recognise particular unnatural amino acids that could be added to the cell-culture media. We created several specific orthogonal pairs that incorporate p-benzoyl-L-Phe, p-acetyl-L-Phe, or p-azido-L-Phe (Figure 2). The system was efficient enough to be able to incorporate these unnatural amino acids into heterologously expressed GPCRs in transiently transfected HEK cells.
The idea of site-directed unnatural amino acid mutagenesis is to introduce a reactive group or probe without loss of function. The ideal site in a GPCR is at a location that does not affect expression level, membrane targeting or function, but is close enough to some site of interest to be useful as a probe of function. We recently introduced azido-Phe into expressed rhodopsin at various locations. We then carried out Fourier-transform infrared difference spectroscopy on purified mutant receptors. We showed that the vibrational signatures of the azido groups change after rhodopsin is activated by light and undergoes a series of progressive conformational changes. Since the vibrational frequency of the azido is sensitive to electrostatic environment, we could correlate the changes in azido FT-IR spectra with protein conformation. This method is particularly informative since the azido stretching frequency is far removed from protein vibrations, which can be recorded simultaneously.
FT-IR spectroscopy may not be practical as a general method to study GPCRs. However, it is possible to encode genetically azido-Phe into other GPCRs. We have recently focused our attention on chemokine receptors, for example. We are using photo-crosslinking approaches to study receptor heterodimerisation. In addition, unnatural amino acids can be tagged with fluorophores or other useful probes. Experiments along these lines are under way.
Functional reconstitution of expressed receptors
In our aim to study reconstituted receptors in membrane bilayer systems as much as possible, we have also developed a nano-scale apolipoprotein-bound bilayer (NABB) system. Based on naturally-occurring high density lipoprotein particles, NABBs are essentially selfassembling membrane scaffold particles. They can be prepared so that they contain one (or perhaps two) functional GPCRs in each particle. The NABBs are small enough that they remain in solution and create a long-term stable environment for GPCRs. One nice advantage of NABB particles is that both topological surfaces of the receptors are accessible simultaneously to the aqueous phase.
Summary
In summary, GPCR signalling complexes are allosteric machines. Agonist receptor ligands outside of the cell induce guanine-nucleotide exchange on a heterotrimeric guaninenucleotide binding regulatory protein (G protein) inside of the cell where the ligandbinding site on the receptor and the nucleotide-binding site and the G protein are on the order of seven to 10 nanometres or more apart. Additional non-canonical signalling pathways facilitate cross talk between linear GPCR-signalling pathways and receptor tyrosine kinase (RTK)-mediated signalling pathways. Receptor phosphorylation by receptor-specific kinases and the binding of various cellular adaptor proteins also regulates receptor desensitisation, internalisation, sequestration and recycling. Receptor oligomerisation, or in some cases hetero-oligomerisation, is thought to modulate receptor cell-surface expression, ligand-binding affinity, and downstream signalling specificity for at least some classes of GPCRs. To understand how GPCRs really work will require the application of new technologies and ongoing interdisciplinary approaches.
References
Ahuja, S., et al., (2009) Helix Movement Is Coupled to Displacement of the Second Extracellular Loop in Rhodopsin Activation. Nat. Struct. Mol. Biol. 16, 168–175.
Banerjee, S., et al., (2008) Rapid Incorporation of Functional Rhodopsin into Nanoscale Apolipoprotein Bound Bilayers (NABB) Particles. J. Mol. Biol. 377, 1067–1081.
Botelho, A. V., et al., (2006) Curvature and Hydrophobic Forces Drive Oligomerization and Modulate Activity of Rhodopsin in Membranes. (2006) Biophys. J. 91, 4464–4477.
Eswar, N., et al. (2003) Tools for comparative protein structure modeling and analysis, Nucleic Acids Res. 31, 3375-3380.
Huber, T., et al., (2008) Structural Basis for Ligand Binding and Specificity in Adrenergic Receptors: Implications for GPCR-Targeted Drug Discovery. Biochemistry 47, 11013–11023.
Mustafi, D., and Palczewski, K. (2009) Topology of Class A G Protein-Coupled Receptors: Insights Gained from Crystal Structures of Rhodopsins, Adrenergic and Adenosine Receptors, Mol. Pharmacol. 75, 1-12.
Overington, J. P., et al. (2006) How many drug targets are there? Nat. Rev. Drug Discov. 5, 993-996.
Periole, X., et al., (2007) G Protein-coupled Receptors Self-assemble in Dynamics Simulations of Model Bilayers. (2007) J. Am. Chem. Soc. 129, 10126–10132.
Rosenbaum, D. M., et al. (2009) The structure and function of G-protein-coupled receptors, Nature 459, 356-363.
Scheerer, P., et al. (2008) Crystal structure of opsin in its G-protein-interacting conformation, Nature 455, 497-U430.
Vogel, R., et al., (2008) Functional Role of the “Ionic Lock” – an Interhelical Hydrogen-bond Network in Family A Heptahelical Receptors. J. Mol. Biol. 380, 648–655.
Ye, S., et al., (2008) Site-specific Incorporation of Keto Amino Acids into Functional G Proteincoupled Receptors Using Unnatural Amino Acid Mutagenesis. (2008) J. Biol. Chem. 283, 1525–1533.
Ye, S., et al., (2009) FTIR Analysis of GPCR Activation using Azido Probes. Nat. Chem. Biol. 5, 397–399.
Ye, S., et al., (2010) Tracking G-protein-coupled Receptor Activation Using Genetically Encoded Infrared Probes. Nature 5, 397–399.
About the Author
Thomas Sakmar
Thomas Sakmar, a biochemist and physician, heads the Laboratory of Molecular Biology and Biochemistry at The Rockefeller University. Dr. Sakmar uses interdisciplinary approaches to study how chemical signals are relayed from the outside to the inside of a cell. This process, known as transmembrane signalling, allows cells and organisms to sense their environments. Much of Dr. Sakmar’s research focuses on vision and on the signalling molecules in the retina, with implications for understanding retinitis pigmentosa, macular degeneration, night blindness, colour blindness and other vision disorders. Investigations in the Sakmar laboratory also explore signalling pathways that play a role in glucose metabolism, inflammation, the brain’s response to the neurotransmitter dopamine, and the ability of the AIDS virus to enter human cells. Dr. Sakmar received a B.A. in chemistry in 1978 from the University of Chicago and went on to earn an M.D. in 1982 from Chicago’s Pritzker School of Medicine. He was an intern and resident in internal medicine at Massachusetts General Hospital and a clinical fellow at Harvard Medical School. In 1985, Dr. Sakmar began postdoctoral research with Nobel Laureate H. Gobind Khorana in the departments of biology and chemistry at the Massachusetts Institute of Technology. He remained at M.I.T. until 1990, when he moved to Rockefeller as an assistant professor and laboratory head. Dr. Sakmar became a tenured professor in 1998 and the University’s Richard M. and Isabel P. Furlaud Professor in 2002. In addition, from 1991 to 2004 he was associated with the Howard Hughes Medical Institute. From February 2002 through August 2003, Dr. Sakmar served as acting president of The Rockefeller University.
Contact the Author
email: [email protected]




