Taking care of innovation
Posted: 22 August 2005 | | No comments yet
The most important factors for ensuring a successful future are innovation and an effective governance structure. Within this model1 in the pharmaceutical industry, the introduction of new technology is one of the key factors. This provides an organisation with efficient and reliable systems to facilitate the incorporation of new scientific concepts and practices and these are vital to ensure the company’s continued success.
The most important factors for ensuring a successful future are innovation and an effective governance structure. Within this model1 in the pharmaceutical industry, the introduction of new technology is one of the key factors. This provides an organisation with efficient and reliable systems to facilitate the incorporation of new scientific concepts and practices and these are vital to ensure the company’s continued success.
The most important factors for ensuring a successful future are innovation and an effective governance structure. Within this model1 in the pharmaceutical industry, the introduction of new technology is one of the key factors. This provides an organisation with efficient and reliable systems to facilitate the incorporation of new scientific concepts and practices and these are vital to ensure the company’s continued success.
New technology is often not directly related to core science. For example, new plate reader automation technology is only important to the core scientist in that it will enable more reliable results to be generated and/or extend the experimental range. It is quite likely that the scientist will have only a limited appreciation of the engineering and very little time to make a proper assessment of the technology.
In house technology groups effectively meet this need and with efficient project management, contribute enormously to improving the cost effectiveness of research. They reduce the burden on the ‘core’ scientist by optimising the choice and application of the technology and providing a knowledge base within a company that avoids ‘reinventing the wheel’ syndrome in disparate core science groups.
Within GSK R&D, the Applied Technology Group (ATG) UK manages as part of its remit the introduction of bespoke large-scale laboratory automation projects. This includes:
- Identifying and evaluating emerging technologies
- Evaluating potential technologies used in other industries and areas
- Optimising speed of implementation
- Comparing and assessing technology providers
The benefits of a group dedicated to this role are readily apparent. Not only do internal customers have a professional group giving full attention to an individual project without the encumbrance of other responsibilities, roles or internal politics, but they also have a group experienced in the rigorous testing of a range of new technologies and in deriving best value from the suppliers. From a customer perspective, the value of experts on their side who are skilled in communication and with a wide range of customers is not to be underestimated. Those communication skills, dependant on the character, behaviour, attitude and leadership of the members of ATG can therefore be as important as their project management expertise2.
The clear focus of this technology group is to improve systems integration methodologies as part of standard project management practise3,4. In general, automation of large-scale processes within GlaxoSmithKline R&D is driven by key factors such as data quality, reduced cost, high throughput and flexibility. This demand has lead to a rethink of project management methodologies – including communication practises within bespoke laboratory automation integration teams.
In general, the choice of method is essential for the successful management of the project life cycle phases5. Methods can be classified as either quantitative or qualitative and are typically used to analyse aspects related to time, capital flow, scope or uncertainty. The phases within the project life cycle are:
- Front-end phase of project: test ideas – feasibility study
- Middle phase of project: development – build/test
- End phase of project: integration of the system into the business environment
Methodology selection for the front-end project phase is less important if the quality of the input data is inferior. It is better to be almost right than precisely wrong6. Hence, it can be concluded that it is the quality of the input to the method that counts. Nevertheless, it is essential to agree on a robust front-end assessment strategy that is usually based on a qualitative method, to allow management of the uncertainty/creativity, inherent in the front-end phase.
On the other hand, there are numerous possible methods available to manage the middle and end phase of a project3. These methods are mainly based on a quantitative approach. Use of quantitative methods demands data which are more readily available in this project phase, for detailed planning. In summary, the complexity of the development and integration task will define the methodology applied.
The success of this approach can be highlighted by describing three case studies. The studies centre around high throughput/low cost SNP genotyping, synthesis technology and systems integration in general.
Case 1 – High throughput/low cost SNP genotyping
Situation
SNP based genotyping is primarily cost and, to a lesser extent, a throughput constrained technology. A number of elegant approaches to solving the current bottlenecks exist. One of these approaches is to miniaturise the reactions conducted to discriminate between the alleles, thereby reducing the reagent consumption and thus cost.
Solution
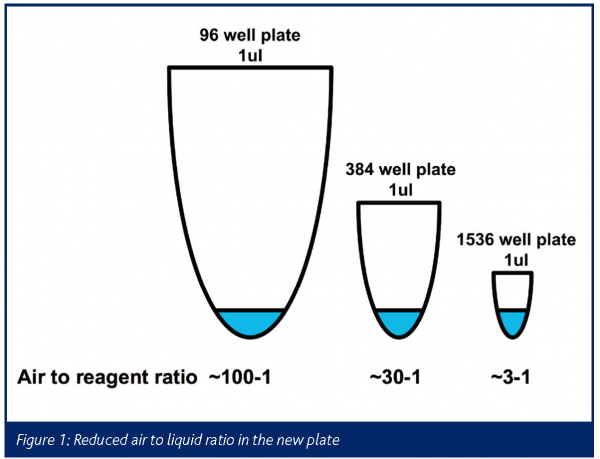
The initial feasibility study included the development of a 1536 polypropylene plate suitable for 1µl reactions and the high temperatures (95°C) required in PCR reactions (Figure 1). The plate was developed by using an injection moulding technique. The injection mould was process modelled and employed novel injection gating techniques to create the final plate.
However, the 1536 PCR plate could not be sealed using 170°C thermal melt, glue backed films as in conventional plate sealers. The high temperatures employed in conventional sealers were shown to rapidly evaporate and degrade the reagents used in PCR.
In order to overcome the sealing issues, technology developed at the University of Warwick, UK was in-licensed and modified further into a Transmission Diode Laser Welding sealing process. This uses a focused 80 micron laser (Infrared 808nm) directed through a clear transmissive layer (50 micron polypropylene film) onto the 1536 microtiter plate, which subsequently heats to create a covalent bond. The process has been shown to cause localised heating, no more than 10 micron below the surface. This was shown to have no effect on the contained DNA sample and PCR reagents. A 1536 plate takes less than 60 seconds to fully seal. In order to routinely use this process we have developed an automated laser sealer as shown in Figure 2.
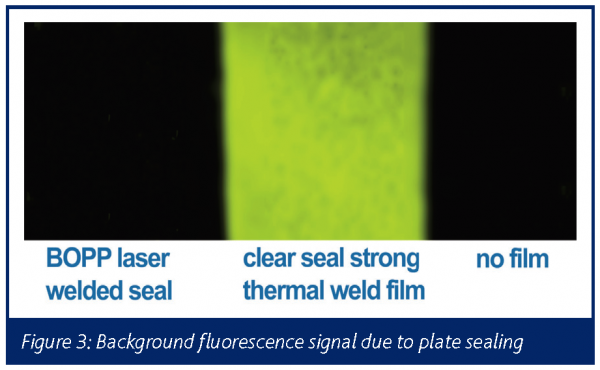
The use of laser welding necessitates the use of identical polymers to create a covalent bond between plate and film. We used 50 micron biaxally orientated polypropylene film (BOPP), which conveyed a number of optical advantages to the fluorescent detection. Fluorescent detection is extremely sensitive, but often background limited.
State-of-the-art sealing technology that is currently in use, reduces light output by up to 50 per cent and increases background significantly. Figure 3 shows a false colour image of the background fluorescence associated with state-of-the-art current sealing technology and the BOPP film used to seal the 1536 PCR plate.
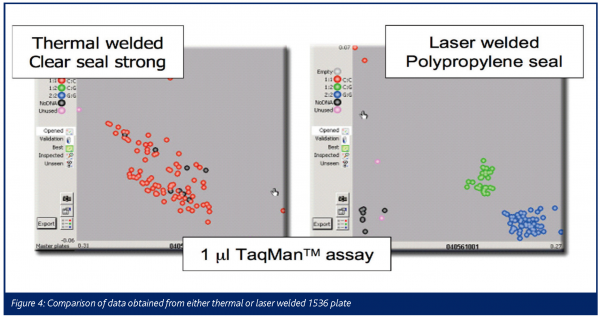
The decreased background and increased signal associated with the use of the BOPP film gives rise to a significant increase in signal to noise of approximately 10:1. The method was tested by analysing two identical 1536 well plates containing 1µl TaqManTM assays on demand TM reagents. The results are shown in Figure 4. One plate was laser welded using BOPP film and the other was thermal welded using conventional clear seal strong film. Both were identically processed and read in a conventional plate reader. Figure 4 clearly identifies the clusters on the laser welded plate with BOPP film which enable the analysis of the assays.
Having developed a very robust genotyping prototype system that:
- reduces edge effects
- gives a robust seal
- no intrinsic fluorescence
- enables novel assays
this was then built into a high throughput pipeline and has been implemented following standard project management principals. A workstation-based approach has been chosen accompanied by a fully integrated LIMS system. This data tracks the whole process from sample ID, primer design, cluster analysis to final data export.
Discussion – Case 1
At GSK we embarked on and completed a round of technology development that has enabled us to robustly miniaturise a number of fluorescent-based SNP Genotyping chemistries to sub-microlitre levels at high-throughput.
The key to the success of this pro-active project was twofold:
- A self-driven project leader with a substantial understanding of the science and technology in this area, gained through long term work relationships with the internal customer department.
- Strong social networks2 of technical experts, both within and outside GSK.
Subsequently, the role of the project leader was to create a virtual team from resources within the host department, as well as including external experts such as consultancies, academics or technical firms.
The essential knowledge that formed the basis for decision making was collected during a well performed front-end assessment. One aspect of systematic front-end assessment is its transfer of knowledge and experience between the virtual team members. The consequence of this continuous transfer of knowledge and experience is a general increase in the competence of all participants.
Case 2 – Synthesis technology
This project was classified as uncertain because it was not possible to define user requirements completely and accurately as they were still evolving. This is not uncommon in pharmaceutical R&D where novel concepts or methods are being evaluated all the time and is particularly the case when the productivity of R&D organisations is under increasing scrutiny7. Such situations must be managed in-house if control is to be maintained concerning what is to be delivered. However, such situations include leverage of resources by using sub-contractors wherever possible.
Situation
Process optimisation is a key stage in the R&D process, identifying routes to manufacture that offer the best profile for purity, yield and cost. Hence, an automated approach was considered for the manual activity of screening the chemical synthesis, which is labour and time intensive as well as an error prone process.
Solution
A fully automated system, Figure 5, where the chemist plans and manages the experiment working in the graphical user interface module of the systems supervisory software.
Imported and user input data are combined and the software generates a schedule for the chemist to approve. Once an experiment has been initiated, progress can be monitored via the user interface, with process data for each of the units being presented in a screen window on request. Samples from the reaction mixture are analysed automatically and chemists can call up spectrums on the systems screen for examination. At the end of the experiment, the systems supervisory software takes the chemist through a procedure, which ensures that the systems are properly closed down and that experimental data is securely stored.
Case 2 – Discussion
The internal client had already introduced a number of innovative systems for process optimisation themselves. However, this project was a team effort, involving both internal and external expertise from several companies in a project managed by ATG. The project management expertise was fundamental in building the system. The project manager put in place a framework that allowed innovation and flexibility whilst retaining control over the specification and costs. Effective communication within the team was achieved through commitment to adequate documentation, managed and distributed by the lead user.
The benefits of maintaining the overall control of the entire project life-cycle for a highly uncertain project are as follows:
- Novel solutions to user requirements
- Protects intellectual property (IP) prior to discussions with external specialists
- Reduces IP and development risks
In this project, existing bottlenecks were identified and appropriate solutions explored by assessing alternative technologies to overcome the limitations of the process.
Case 3 – Systems Integration
The two projects described within this section fall within the low risk category because their user requirements can be completely and accurately specified. Hence, they ought to be contracted out unless there are specific reasons in favour of an in-house managed project, e.g. a unique in-house expertise and experience, IP issues or possibly speed of response.
Situation
A systems integrator is responsible for the overall integration of either an automated platform or the dissemination of vendor’s products into a system. An automated system designed to perform repeatable assays to generate high quality data was required. The assays are programmed and the system is controlled via a time critical scheduler called Overlord scheduler. At the same time a different systems integrator was required so that chemical processes could be characterised in a pilot plant environment.
Solution
One appropriate systems integrator was selected to develop the automated repeatable assays application. The system was split into a fully automated cell, where all time critical assay steps are carried out and a manual cell where users can carry out the remainder of the assay under sterile conditions. The software allowed multiple users to run different assays at the same time on the automated system controlled by the Overlord scheduler.
An alternative systems integrator suitable for pilot plant design was selected to build a mini batch plant. The fully automated rig was highly flexible, such that chemical processes could be characterised prior to scale-up work.
Case 3 – Discussion
It can be concluded that the systems integrator and internal project manager selection are of key importance for the successful completion of multiple projects. However, it should be pointed out at this stage that the perfect system integrator may not exist because project implementation in the pharmaceutical R&D environment is not trivial. Therefore, from a leadership perspective, be prepared to grow your project manager as well as systems integrator and use the tendering process to identify potential. Furthermore, even if the systems integrator and project manager perform poorly on one project, do not be too quick to discard them.
Applied Technology has the infrastructure in place, in the form of relationships with capable companies, to act as a multiple project manager and assign external systems integrators for projects within GSK. It is the GSK project manager’s responsibility to specify the user requirements as well as the project purpose and goal with the internal client and subsequently select the most appropriate systems integrator by applying a rigorous tendering process. In our experience, one GSK project manager is stretched with managing three integration projects, at any one time, depending on the complexity of the project portfolio.
Conclusion
Applied Technology’s particular contribution within this model is the ability to innovate and develop innovative technologies where these technologies extend the capabilities of GSK R&D through a successful technology project management framework. In embracing this role the department promotes good practice in the supply of laboratory instrumentation and systems; establishes effective partnerships with industry and the academic world and brings the most appropriate resources to bear on GSK project needs.
Acknowledgement
I would like to thank my colleagues Stuart Anscombe, Jon Curtis and Sid Shaikh for their contributions to this article.







References
- Yamasaki, H., New Dimension in R&D Management. Presentation at the 12th international conference on management of technology, IAMOT, Nancy, France, May 2003
- Keller, T.H., The role of management structure in the absorption and diffusion of scientific knowledge. M.Phil. Dissertation, University of Cambridge, Judge Institute of Management Studies, England, July 1999
- www.ipma.ch (International project management association), www.apm.org.uk (Association for project management), www.pmi.org/ (Project Management Institute)
- Harrison F., Lock D., Advanced Project Management; A structured approach. Grower Publishing Limited, UK, 2004
- Grey S., Practical Risk Assessment for Project Management. John Wiley & Sons, UK, 1995.
- Hartman G., Risk is still a four letter word. Stoddart Publishing Co. Limited, Canada, 2000
- Brown L., Grundy T., Project Management for the Pharmaceutical Industry. Gower Publishing Limited, UK, 2004




