Opportunities for Rapid Microbial Methods
Posted: 2 February 2006 | | No comments yet
Since the 2000 publication of the PDA Technical Report Number 33 Testing Methods, rapid microbial methods (RMM) have not lived up to their promise for changing routine Quality Control microbiological testing in the pharmaceutical industry. With the next generation of rapid microbial methods emerging from the R&D laboratories of instrument manufacturers, this article will discuss the obstacles to the successful implementation of the currently marketed technologies and strategies to overcome these obstacles.
Since the 2000 publication of the PDA Technical Report Number 33 Testing Methods, rapid microbial methods (RMM) have not lived up to their promise for changing routine Quality Control microbiological testing in the pharmaceutical industry. With the next generation of rapid microbial methods emerging from the R&D laboratories of instrument manufacturers, this article will discuss the obstacles to the successful implementation of the currently marketed technologies and strategies to overcome these obstacles.
Since the 2000 publication of the PDA Technical Report Number 33 Testing Methods, rapid microbial methods (RMM) have not lived up to their promise for changing routine Quality Control microbiological testing in the pharmaceutical industry. With the next generation of rapid microbial methods emerging from the R&D laboratories of instrument manufacturers, this article will discuss the obstacles to the successful implementation of the currently marketed technologies and strategies to overcome these obstacles.
Despite the huge 2004 investment of US$44.5 billion in R&D compared to $450 billion in sales (10% of sales), global pharmaceutical companies are struggling to launch new drug products with blockbuster potential in the face of the patent expirations of their current revenue-generating products (Truelove, 2005). Many major pharmaceutical companies are restructuring their manufacturing operations in an attempt to maintain their profitability.
On November 28, 2005 Merck & Co., Inc announced a global restructuring program to reduce the company’s cost structure, increase efficiency and enhance competitiveness. The initial steps will include the implementation of a new supply strategy by the Merck Manufacturing Division, which is intended to create a leaner, more cost-effective and customer-focused manufacturing model over the next three years. As part of a global restructuring program, Merck expects to eliminate some 7,000 positions in manufacturing and other divisions worldwide, representing about 11% of its global work force, by the end of 2008. Merck intends to sell or close five of its 31 manufacturing facilities worldwide and to reduce operations at a number of other sites. Merck stated that they will implement a new supply strategy by creating a global facility network that combines the best of Merck manufacturing with the manufacturing capabilities of key external suppliers, introducing a new production system based on lean manufacturing principles, and developing a new approach to product commercialisation to enable accelerated delivery of Merck’s research pipeline through the launch phase. Merck plans to reduce the time from customer order to manufacturing and significantly improve the production system to reduce manufacturing costs, inventory and cycle time throughout its network. A pilot program now under way at Merck’s pharmaceutical manufacturing site in Arecibo, Puerto Rico, is delivering a 50% reduction in on-site cycle time and on-site inventory reduction of greater than 30%.
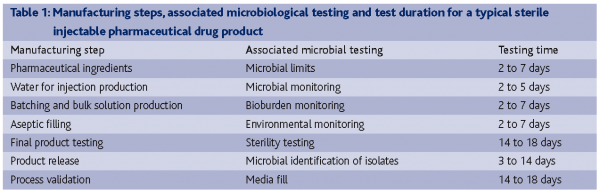
In the new supply strategies that are so popular with global pharmaceutical companies, what role can improved laboratory efficiency with RMMs play? If we examine the microbial testing associated with the manufacture and release of a sterile injectable pharmaceutical product, it becomes apparent that growth-based microbial testing is the rate-limiting QC testing activity, not batch-record review or chemical testing (Table 1).
Benchmarking QC testing laboratories
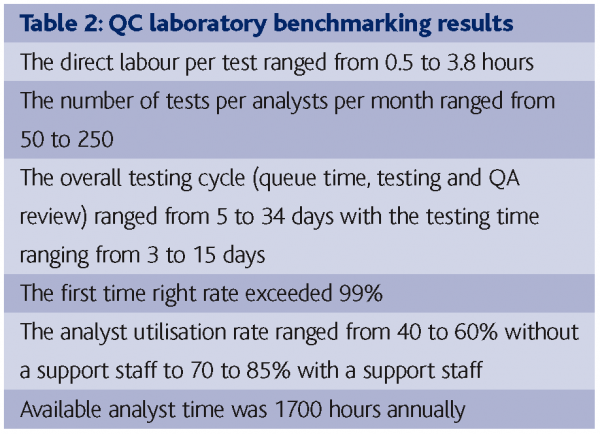
Bublitsky and Howard (2005) reported the benchmarking of 15 labs in the biopharmaceutical industry as part of a lean manufacturing study that give an insight into QC laboratory operations. The parameters benchmarked were:
- Staffing
- Cycle time
- Analyst availability and utilisation
- Productivity
- Quality
- Support systems
Table 2 contains a summary of the benchmarking results. Points to note were the relative low labour input per test; the wide disparity between laboratories; the high ‘first time right’ rate; the low efficiencies in scheduling tests; reviewing the results, release product and the value of support staff in increase analyst utilisation. Clearly the microbiological testing – due to the extended incubation times of growth-based detection, enumeration and identification methods – is an area that would be improved by the application of RMMs. However, it seems possible to achieve 20-30% improvements in cycle times through better scheduling, resource allocation and QA release efficiencies so these can be considered low hanging fruit compared to RMMs.
Classification of RMM
As pharmaceutical microbiologists, our objectives are to determine which microorganisms, if any, are in pharmaceutical ingredients, intermediates, the plant environment or drug products, how many microorganisms and which microorganisms they are, to make decisions to release product to the market. The test methods are classified as detection, screening, enumeration and identification. Examples are sterility testing (detection), absence of specified microorganisms (screening), microbial count (enumeration) and microbial identification (identification).
The classification systems for rapid methods proposed in the PDA Technical Report are based on how the technology works, e.g., growth of microorganisms, viability of microorganisms, presence/absence of cellular components or artifacts, nucleic acid methods, traditional methods combined with computer-aided imaging and combination methods (Moldenhauer, 2004).
- Growth-based technologies: These methods are based on measurement of biochemical or physiological parameters other than turbidity or colony formation that reflect the growth of the microorganisms. Examples include: ATP bioluminescence, colorimetric detection of carbon dioxide production and measurement of change in head- space pressure, impedance, advanced imaging and biochemical assays.
- Viability-based technologies: These types of technologies do not require growth of microorganisms for detection. Differing methods including vital staining and fluorogenic substrates are used to determine if the cell is viable and if viable cells are detected, they can be enumerated. Examples of this technology include solid phase cytometry and flow fluorescence cytometry.
- Cellular component or artifact-based technologies: These technologies look for a specific cellular component or artifact within the cell for detection and/or microbial identification. Examples include: fatty acid profiles; Matrix Assisted Desorption Ionised – Time of Flight (MALDI-TOF) mass spectrometry; enzyme linked immunosorbent assay (ELISA); fluorescent probe detection and bacterial endotoxin-limulus amebocyte lysate test.
- Nucleic acid-based technologies: These technologies use nucleic acid methods as the basis for operation for detection, enumeration and/or identification. Examples include: DNA probes, ribotyping/molecular typing, polymerase chain reaction (PCR) and base sequencing.
Selection of the appropriate RMM
What are the advantages in developing, validating and implementing RMMs? They include reducing the product release cycle time; reducing raw material; work in progress and finished product inventories; improving the quality of the microbial testing; automating the microbial testing; electronic capture of test data and information creation; the ability to start investigations earlier in response to out-of-specification (OOS) results; potentially reducing risk of microbial contamination of products and improving the manufacturing processes and the professional enrichment within the laboratory. Given these advantages why has the implementation of RMMs been so slow? The obstacles to the implementation of RMMs include regulatory risk adverse positions taken by pharmaceutical companies; higher capital and unit testing costs; technical obsolescence in the microbiological testing laboratories and lack of resources within the microbiology testing labs to implement RMMs.
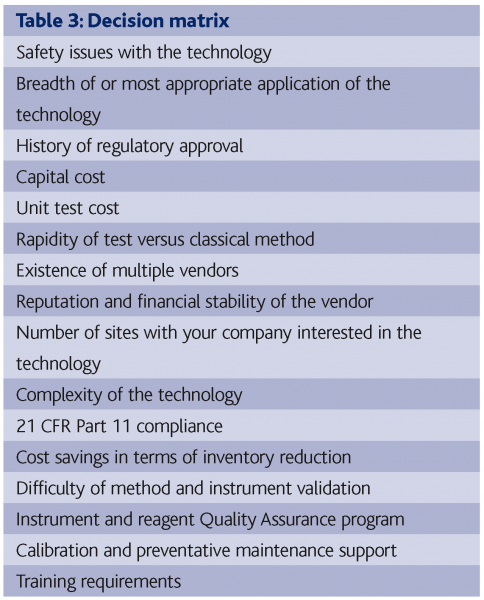
A major predicator for success in the implementation of RMMs is the selection of the best technology for a particular application. Many companies have soured on RMMs because they did not afford sufficient attention to this step. Table 3 contains a list of pertinent parameters that need to be considered as a decision matrix when making an RMM selection.
Validation strategies
The users’ requirements document defines the functional and operation specifications of the assay and instrumentation and details the justification for the selection of the supplier. For example, a microbial enumeration method may be required to electronically record equivalent counts of aerobic microorganisms to the compendia method, within 18 hours of incubation and be non-destructive.
The validation protocol will consist of documents – namely the Installation Protocol (IP), the Operation Qualification (OQ) and the Performance Qualification (PQ). The IQ is the verification and documentation that the testing equipment was supplied and installed as specified by both the user and supplier. For example, that the system received matches that of the purchase order and is installed within a laboratory that meets specified ambient temperature and humidity requirements, has a proper power supply and has the appropriate preventive maintenance and calibration program.
The OQ confirms that the system functions as specified and operates correctly every time. For example, instrumentation responses to computer functions; reagents are dispensed correctly; incubators operate within the specified temperature range; QC organisms perform as expected and database accurately stores and retrieves results.
The PQ confirms that the lab equipment consistently performs as required on actual samples. For example, the system gives consistently equivalent, i.e., as good or better results to the official microbial test, meeting all method validation criteria, e.g., accuracy, precision, specificity, linearity and repeatability. It should be emphasised that citing peer-reviewed scientific publications and supplying vendor-generated studies may be used in regulatory applications, so it is not necessary to repeat pre-existing work.
A comprehensive validation plan should be written to provide a road map for the assay and instrument validation. A validation protocol must be written and reviewed and approved by your Quality Control Unit.
The protocol should include intent of the study, methods, equipment and materials, documentation to capture the results, acceptance criteria and provision for deviation from the protocol.
Regulatory approval
Your company has evaluated, developed and validated an RMM for the testing of drug products, but how will you get approval to implement the RMM? Remember, regulatory agencies approve drug products and not testing methods. With the FDA, RMMs may be included in post-approval changes in chemistry, manufacturing and controls (CMC) sections for existing products may be submitted in new drug applications (NDAs), abbreviated new drug applications (ANDAs), or supplements to these applications. Also, RMMs may be filed in Comparability Protocols or using the Process Analytical Technology (PAT) Initiative.
What is a comparability protocol and why is it the preferred method for handling RMMs? A comparability protocol is a plan for assessing the effect of specific CMC changes on a specific drug product(s) related to the safety and effectiveness of the product(s). It describes the changes covered under the protocol and specifies the tests and studies that will be performed, including the analytical procedures that will be used, and acceptance criteria to demonstrate that specified CMC changes do not adversely affect the product. Although the submission of a comparability protocol is optional, the FDA currently prefers this type of submission. When an application containing the comparability protocol is approved, the FDA can reduce reporting category for future reporting of CMC changes covered by the approved comparability protocol. With a comparability protocol, the FDA is less likely to request additional information to support changes made under the protocol. It could allow an applicant to implement CMC changes and place a product in distribution sooner than without the use of a comparability protocol.
The comparability protocol should contain a description of the RMM and how it will be applied; reference to a DMF submitted by the supplier; copies of peer-reviewed scientific articles; a detailed experimental protocol; statistical treatment of data; the acceptance criteria and the reporting category to be used for future supplements.
With regard to PAT applications, contact OPS/CDER/FDA to discuss a proposal and/or submission. Each PAT submission will be assigned to PATRIOT (PAT Review and Inspection Team) consisting of CMC reviewers from different divisions, compliance officers, industry consultants and field investigators.
In May 2004, the FDA granted GlaxoSmithKline (GSK) approval to use the Pallchek™ Luminometer as part of the quality control process for a prescription nasal spray in its Parma, Italy facility. The Pall Corporation rapid test enables GSK to release product to market up to four days earlier than before. GSK was the first pharmaceutical company to obtain approval to release a prescription product to market using a rapid detection technology under the FDA PAT program. In February 2004, the FDA approved the supplement to the Genzyme Biosurgery biologics license application for Autologous Cultured Chondrocytes to utilise the BacT/Alert automated microbial detection system for the release of product based on interim negative results at approximately day three of a 14-day sterility test.
Genzyme committed to perform side-by-side testing on contaminated clinical samples using the BacT/Alert and traditional sterility test for one year following approval of this supplement.
European regulators also support RMMs. In 1997, the UK Medicines and Healthcare Products Regulatory Agency (MHRA) approved the use of Celsis Rapiscreen for screening non-sterile pharmaceutical and OTC drug products for an absence of microorganisms by Wyeth. In October 2001, the UK MHRA approved the use of the ScanRDI for microbial monitoring of pharmaceutical grade water by GSK.
Key documents in support of RMM implementation in the pharmaceutical industry are found in Table 4.
Promising new technologies
Clinical microbiology laboratories are implementing real-time reverse transcription PCR to determine the microbial load and identify the infectious agents from different clinical specimens, as well as for microbial types for which routine-growth-based culture and microscopy methods are inadequate due to fastidious culture requirements and/or slow growth (MacKay, 2004). Primers and probes may be selected to detect, enumerate and identify groups of microorganisms or specific microorganisms. PCR amplifications may go through 36 to 48 cycles in the order of 20 minutes, which is a single generation time for a growth-based method with the number of cycles to reach a threshold level being a measure of the magnitude of the microbial level in the original sample. Furthermore, the amplified nucleic acid may be isolated and subjected to base sequencing to identify the microorganism within the sample.





References
PDA Technical Report No. 33 The Evaluation, Validation and Implementation of New Microbiological Testing Methods. PDA J. Pharm. Sci. & Technol. 54 Supplement TR#33 (3) 2000
Truelove, C 2005 Annual Report – Introduction: Beyond the Image Problems Med Ad News 24 (9) 4, 6, and 8
Whitehouse Station, N.J.–(Business Wire)–Nov 28, 2005 -Merck & Co., Inc. www.pharmalive.com/news/index
Bublitsky, T and A. Howard (2005) Benchmarking QC Operations Pharm Tech. Europe September 2004
Moldenhauer, J 2005 Rapid Microbiological Methods and the PAT Initiative BioPharma International September 15 issue
MacKay, I M 2004. Real-Time PCR in the Microbiology Laboratory. Clin. Microbiol. Infect. 10 190-212









