Advances in fast ion chromatography
Posted: 24 March 2006 | | No comments yet
Ion chromatography (IC) allows the determination of trace ions using low capacity high efficiency columns possessing fixed ion exchange sites. Combined with suppressed conductivity detection, IC yields parts-per-billion detection of the seven common inorganic anions (F-, Cl-, NO2-, Br-, NO3-, HPO42- and SO42-) and to a lesser extent carboxylic acids, the six common cations (Li+, Na+, NH4+, K+, Mg2+ and Ca2+) and small amines1, 2.
Ion chromatography (IC) allows the determination of trace ions using low capacity high efficiency columns possessing fixed ion exchange sites. Combined with suppressed conductivity detection, IC yields parts-per-billion detection of the seven common inorganic anions (F-, Cl-, NO2-, Br-, NO3-, HPO42- and SO42-) and to a lesser extent carboxylic acids, the six common cations (Li+, Na+, NH4+, K+, Mg2+ and Ca2+) and small amines1, 2.
Ion chromatography (IC) allows the determination of trace ions using low capacity high efficiency columns possessing fixed ion exchange sites. Combined with suppressed conductivity detection, IC yields parts-per-billion detection of the seven common inorganic anions (F-, Cl-, NO2-, Br-, NO3-, HPO42- and SO42-) and to a lesser extent carboxylic acids, the six common cations (Li+, Na+, NH4+, K+, Mg2+ and Ca2+) and small amines1, 2.
Within the pharmaceutical industry, IC finds a wide array of applications3. The following are some examples:
- To characterise pharmaceuticals, their metabolites and impurities – particularly in the early stages of research
- To identify and quantify counter ions
- As an alternative to atomic absorption spectroscopy for the determination of inorganic cations
- To determine non-UV active aliphatic amines
Introduction to ion chromatography
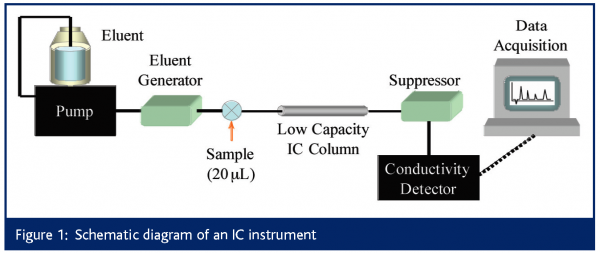
The primary suppliers of IC instrumentation are Dionex4, Metrohm5 and Alltech6. Regardless of the supplier, an IC system appears much like an HPLC, as shown in Figure 1. However, there are a number of subtle but important differences. Firstly, the entire flow path (pump, injector, column, tubing and detector cell) is metal-free, typically constructed of polyetheretherketone (PEEK).
Dedicated or process IC units typically use isocratic pumps, while higher end IC systems can perform gradient elution. Eluent can either be prepared manually or electrolytically generated on-line7. Electrolytic eluent generation offers greater convenience and lower limits of detection. The flow range of IC pumps mirrors that of modern microbore (2 mm i.d.) or conventional (4.6 mm i.d.) column systems, but no capillary IC systems are commercially available, although this is a current topic of research8. Degas modules are available, however they are not as common or as necessary as in RPLC as pure aqueous eluents are typically used in IC.
The typical injection volume is 20 mL, with 1 μL injections or preconcentrator columns used for trace (sub ppb) analysis9. Temperature control is recommended to maintain retention time reproducibility and can significantly improve the baseline, which is particularly important for trace analysis. Preferred eluents for anion separations are salts of weak acids (pH>7) such as (in order of increasing strength): hydroxide (-OH), bicarbonate (HCO3-) and carbonate (CO32-). The mixture of bicarbonate and carbonate ions is often used as a powerful eluent for the separation of both singly and doubly charged analytes in a single chromatogram.
Due to the alkaline nature of IC eluents, the stationary phases in IC are usually polymer-based ion exchangers. Conductivity detection provides a universal detection mode for ionic species. However, since the eluent in IC is conductive itself, it causes a large conductivity background, worsening limits of detection. To reduce this background, either low concentrations of weakly conductive eluent are used with low capacity columns (non-suppressed IC) or an eluent suppressor is added to the system between the column and the detector (suppressed IC).
Eluent suppression in anion exchange IC improves the detection limits of anions from the parts-per-million (ppm, μg/mL) to the parts-per-billion (ppb, ng/mL) level through two processes. First, the eluent is converted to a lower conductive form to decrease the conductivity background. Second, suppression increases the conductivity of the analytes. Details of suppressor development and a description of commercially available suppressors are presented in a recent review10.
High speed IC
In recent years, high-speed separations have been a major area of focus in reversed-phase liquid chromatography. This is not surprising given that laboratories are under constant pressure to improve sample throughput and boost productivity. The three primary approaches to improving the throughput of RPLC are: elevated temperatures; monolithic columns and short columns containing small particles. This article will review the effectiveness of these approaches to improving the speed of IC separations.
High temperature IC
Temperature is now being used as an additional variable to control retention and selectivity in RPLC12-14. At temperatures above 140°C, water has the characteristics of an organic solvent in terms of dielectric constant, and the solubility of hydrophobic molecules is greatly improved relative to that at room temperature. Further, elevated temperatures reduce the viscosity of the mobile phase. This reduces the backpressure of the column and improves the mass transfer within the column – both factors that enable faster flow velocities to be used to achieve rapid separations. Finally, elevated temperatures also reduce the peak tailing associated with cationic drugs
However, until recently, the use of temperature to modify selectivity in IC has received relatively little attention. For anion exchange separations, the effect of temperature on selectivity can be quite complex16,17. Many ions show a beneficial decrease in retention with temperature, but some – most notably the late eluting SO42- – increase in retention, limiting improvements in the analysis time. Furthermore, the quaternary ammonium sites of anion exchange columns are prone to degradation above 60°C under the alkaline eluent conditions used in IC. Thus, elevated temperatures offer little opportunity to increase the throughput of anionic separations.
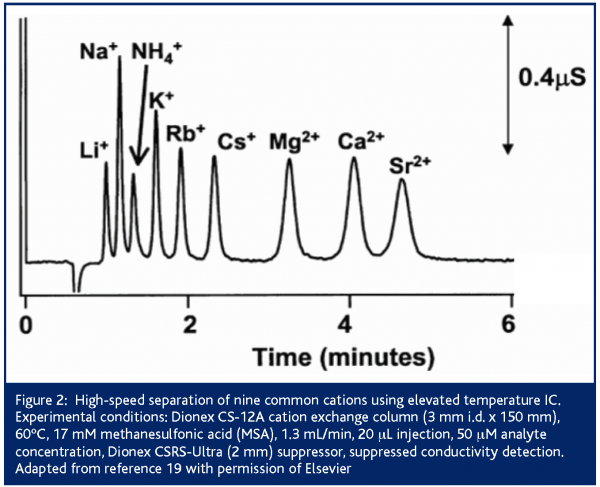
Conversely, temperature offers significant opportunities for cationic separations. Cation exchangers are temperature stable and retention in cation exchange chromatography typically decreases with increasing temperature18. Increasing the temperature of a Dionex CS12A column to 60°C allows nine cations to be separated in under five minutes compared to 12 minutes at room temperature (Figure 2)19. Further, temperature affects the retention of different classes of cations (i.e., alkali metal, alkaline earth metal or amine) to differing extents18,20. Therefore, for both speed and selectivity, elevated temperatures are commonly used for IC separations, particularly those involving both inorganic cations and amines.
Monolithic columns
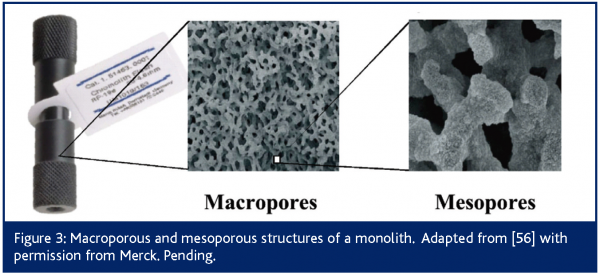
Monolithic columns, consisting of continuous beds with through pores of organic or inorganic matrix, have found increasing applications for HPLC separations21-23. Silica monolith columns have been most extensively used in fast IC methodologies24. These columns exhibit a different internal geometry than columns packed with particles, as illustrated in Figure 3. Monoliths are obtained through a sol-gel process and enclosed in a PEEK sheath21,23 . In essence, silica monoliths consist of a rod of stationary phase (i.e., silica) with typically 13 nm mesopores for retention and 2 μm macropores (larger channels) for through-flow, although these pore sizes can be independently adjusted21. Silica monolithic columns are equivalent to 3 µm particulate columns in terms of band broadening and to 15 µm particles with regard to permeability25. In other words, these columns exhibit high efficiency while generating minimal pressure drop. These characteristics enable the use of very high flow rates (as high as 15 mL/min) that increase sample throughput to 3-10 times that of conventional RPLC columns21,26. For instance, quantitative LC/MS determination of methylphenidate, a central nervous stimulant, and its de-esterified metabolite ritalinic acid in rat plasma was achieved in 15 s using a monolithic RPLC column and a flow rate of 3.5 ml/min27. That translated into eight 96-well plates (768 samples), including standards, QC samples, blanks and double blanks, being analysed in 3 hr 45 min.
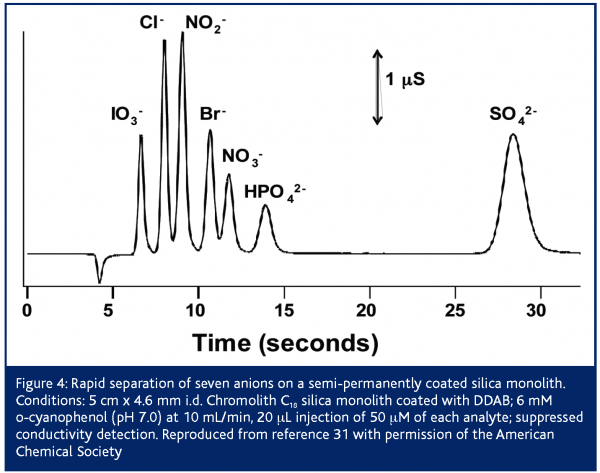
Initially silica monoliths were commercially available only as bare silica, C8 and C18 bonded phases28,29. Such reversed-phase columns are not well suited to the separation of inorganic anions, but can be converted into an anion exchange column by dynamic or semi-permanent coating with a hydrophobic cation. Ion interaction chromatography using a mobile phase of tetrabutylammonium (ion pair reagent) and phthalate (eluent) were able to separate eight anions in 15 s using a flow rate of 16 mL/min30. However, detection limits using non-suppressed conductivity or indirect UV absorption detection were poor (0.1-4 and 0.4-20 ppm, respectively). Coating the C18 monolith with a hydrophobic surfactant such as didodecyldimethylammonium bromide (DDAB) or cetyltrimethylammonium chloride (CTAC) yields a semi-permanent coating. Figure 4 shows the separation of seven inorganic anions in 30 s using a DDAB coated 5 cm C18 monolith31. The stability of the coating enabled suppressed conductivity detection to be used, which yielded detection limits of 4-30 ppb. Success in achieving such rapid separations using elevated flow rates such as the 10 mL/min in Figure 3 requires careful attention to the impact of the extra column components of the chromatograph32. In particular, components that exhibit flow rate dependencies on the amount of band broadening (e.g., connecting tubing and detector response time) must be given the most attention in high-speed separations31,32. Another advantage of semi-permanent coated columns is the ability to easily adjust the column capacity. For instance, high ion exchange capacities are essential for the analysis of analytes within high ionic strength matrices. Ito et al. were able to determine nitrite and nitrate within seawater in 3 min by coating a 5 cm C18 monolith with CTAC to an ion exchange capacity of 400 μequiv33 . However, while these separations are impressive, the semi-permanently coated columns have drawbacks. Primarily, the surfactant gradually washes off the column causing a gradual change in retention times (10% change after 12 hours at 5 mL/min31). To achieve long term reproducibly retention times it is necessary to regularly regenerate the surfactant coating. Alternately, a small amount of surfactant can be continually introduced to the column either in the eluent34 or from a surfactant-saturated pre-column located before the injector35.
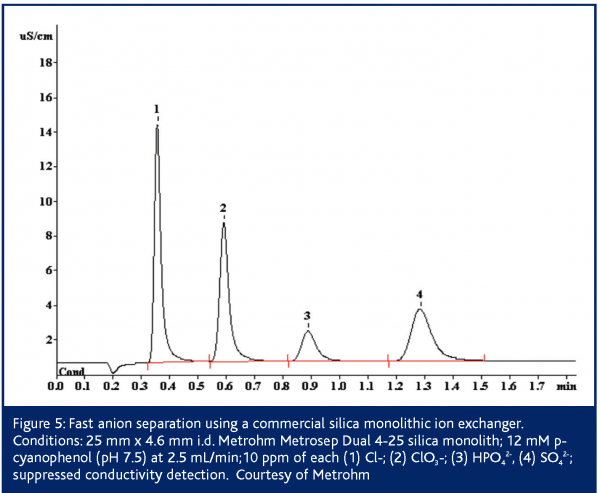
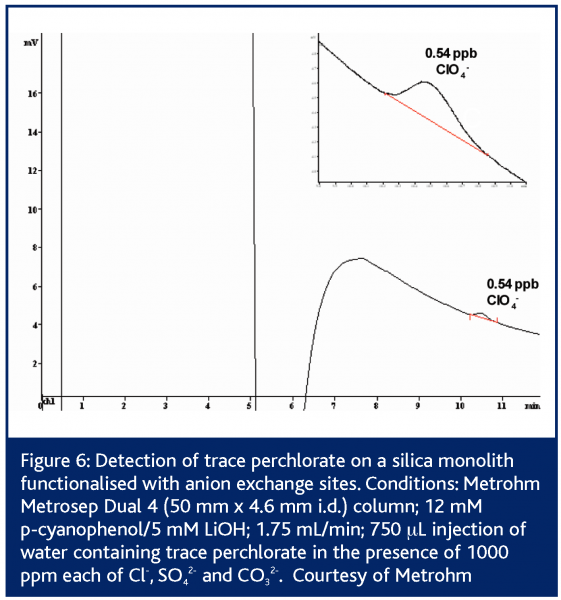
In European Pharmaceutical Review, Issue 1 2005,24 Paull and Nesterenko detailed numerous custom bonded phases for ion analysis including lysine bonded phases for fast (<2 min) separation of UV-absorbing anions36 and iminodiacetate bonded phases for alkaline earth metals37-39. Since then, Metrohm has introduced the Metrosep Dual 4 series of silica monoliths functionalised with anion exchange sites40. These columns possess 2 μm through-pores and 13 nm mesopores. The columns exhibit low backpressures even up to flow rates of 5 mL/min. The Metrosep Dual 4 comes in three lengths/capacities: 25 mm/23 μequiv; 50 mm/45 μequiv and 100 mm/90 μequiv. Separation of simple anion mixtures can be performed on the 25 mm Metrosep Dual 4 in less than 2 min (Figure 4). Some simple separations such as nitrite/nitrate complete in 45 s – faster than some flow injection determinations. The 50 mm Dual 4 is applicable to a wider array of applications as its higher capacity makes it less sensitive to matrix effects. Silica based columns are however limited to pH 2-8. This restricts the above methods to eluents possessing only a -1 charge. Such eluents are effective for singly charged analytes, but the retention times for multiply charged species are longer than ideal 1 (Figure 3). The high permeability of monolithic columns enables shorter analysis times to be achieved through the use of flow gradients where the flow rate is increased during the run, or double gradients in which both the eluent pH and the flow rate are adjusted during the separation39,43,44.
The use of polymeric monoliths would circumvent the pH limitation of silica. Polymeric monoliths have been widely used in RPLC and biomolecule separations22. Unfortunately, their micro-pore structure yields only poor efficiencies in the separation of inorganic ions45,46. Anion exchange monoliths have been prepared by coating methacrylate monoliths with quaternary amine functionalised latex particles to form an agglomerated phase47,48. Capillary columns have been used for separations of inorganic anions47 and carbohydrates48. These preliminary separations suffer from only modest speed and efficiency, but are an approach to watch in future.
Small particles
Improvements in analysis time in HPLC have been achieved through a shortening of the column with a simultaneous decrease in particle size. Column efficiencies have been greatly enhanced through reduction of the particle diameter from 100 μm in the 1950s down to 1.7 μm today49. With a 15 cm column, 10,000-25,000 plates can be obtained using the most popular particle size of 5 and 3 μm. The same columns packed with sub-2 mm particles can generate more than 30,000 plates but require the use of ultrahigh pressure liquid chromatography (UPLC) instrumentation since the pressure drops are well above 5,000 psi.
A similar trend in IC column technology is observed with the smallest particles being approximately 5 μm50. This relatively large particle size is the main reason why conventional IC has not entered the realm of sub-minute separations. At room temperature, the separation of the common anions with a 10 cm packed polymeric column generally takes 8-10 minutes9.
The situation is changing however. Significant reductions in analysis time have been demonstrated by Connolly and Paull51-53. They employed a 30 x 4.6 mm i.d. silica-based reversed-phase column packed with 3 μm particles for the ion-interaction separation of nitrite and nitrate in under 45 seconds. Refinements in the technology resulted in a 2.5-minute separation of 9 common anions53.
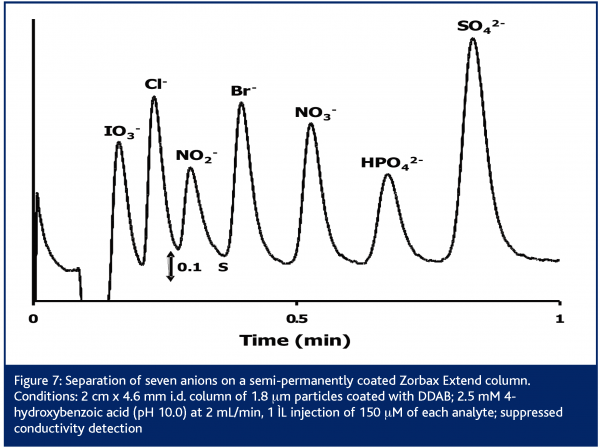
Recently, Agilent Technologies has commercialised a line column packed with 1.8 μm particles of silica-based Zorbax phases54. One of these columns, the Zorbax Extend, is stable up to pH 11.5 and is of particular interest for IC. Figure 7 shows the separation of anions using an Extend column with a 4-hydroxybenzoic acid eluent at pH 10.055 . At this elevated pH, the eluent is doubly charged which allows for faster elution of the -2 ions. The usual elution gap between the -1 and -2 ions (Figure 3) is then reduced, increasing analysis speed. Short 2 cm columns generate moderate pressures and are sufficient to obtain satisfactory resolution. The use of an UPLC system is unnecessary.
Conclusions
Evolution does not occur gradually, but rather its path involves long periods of little change punctuated by short periods of dramatic change2. IC is clearly undergoing a period of rapid change. New developments within HPLC are permeating IC – enabling ever faster separations. Fast IC separations based on monolithic columns have now been commercialised. This will dramatically increase the use of fast IC, which will spur on further developments. These will focus on: improving the column pH stability; improving the selectivity and capacity of fast IC columns so that more complex samples can be analysed and making the transition to capillary IC – both for the dramatic reduction in solvent consumption and for greater compatibility with the rapidly expanding monolithic technologies. It is indeed an exciting time in the field of IC.







References
- Lucy CA, Hatsis P: Ion Chromatography. In: Heftmann E, ed. Chromatography, 6th ed, vol A. Amsterdam: Elsevier, 2004; Ch. 4.
- Lucy CA: Evolution of ion-exchange: from Moses to the Manhattan Project to Modern Times. Journal of Chromatography A 2003; 1000(1-2): 711-724.
- Weiss J: Ion Chromatography in the Pharmaceutical Industry. Handbook of Ion Chromatography, 3rd ed, vol 2. Weinheim: Wiley-VCH, 2004; 756-772.
- www.dionex.com.
- www.metrohm.com/products/ic.html.
- www.alltechweb.com.
- Liu Y, Srinivasan K, Pohl C, Avdalovic N: Recent developments in electrolytic devices for ion chromatography. Journal of Biochemical and Biophysical Methods 2004; 60(3): 205-232.
- Kubin P, Dasgupta PK: Capillary ion chromatography. J. Sep. Sci. 2004; 27: 1441-1457.
- Weiss J: Handbook of Ion Chromatography, 3rd ed, Vol. 1 and 2. Weinheim: Wiley-VCH, 2004.
- Haddad PR, Jackson PE, Shaw MJ: Developments in suppressor technology for inorganic ion analysis by ion chromatography using conductivity detection. J. Chromatogr. A 2003; 1000: 725-742.
- Weiss J: Ionenchromatographie, 3rd ed. Weinheim: Wiley-VCH, 2001.
- Dolan JW: Temperature selectivity in reversed-phase high performance liquid chromatography. J. Chromatogr. A 2002; 965: 195-205.
- Greibrokk T, Andersen T: High-temperature liquid chromatography. J. Chromatogr. A 2003; 1000: 743-755.
- Zhu C, Goodall DM, Wren SAC: Elevated Temperature HPLC: Principles and Applications to Small Molecules and Biomolecules. LC-GC Europe 2004(17): 530-540.
- Albert M, Cretier G, Guillarme D, Heinisch S, Rocca JL: Some advantages of high temperature for the separation of pharmaceutical compounds with mass spectrometry detection. Journal of Separation Science 2005; 28(14): 1803-1811.
- Hatsis P, Lucy CA: Effect of temperature on retention and selectivity in ion chromatography of anions. Journal of Chromatography A 2001; 920: 3-11.
- Barron L, Nesterenko PN, Paull B: Use of temperature programming to improve resolution of inorganic anions, haloacetic acids and oxyhalides in drinking water by suppressed ion chromatography. Journal of Chromatography A 2005; 1072(2): 207-215.
- Hatsis P, Lucy CA: Evaluation of column temperature as a means to alter selectivity in the cation exchange separation of alkali metals, alkaline earth metals and amines. Analyst 2001; 126(12): 2113-2118.
- Chong J, Hatsis P, Lucy CA: H igh-speed ion chromatographic separation of cations at elevated temperature. J. Chromatogr. A 2003; 997: 161-169.
- Rey MA, Pohl CA: Novel cation-exchange stationary phase for the separation of amines and of six common inorganic cations. J. Chromatogr. A 1996; 739: 87-97.
- Cabrera K: Applications of silica-based monolithic HPLC columns. Journal of Separation Science 2004; 27(10-11): 843-852.
- Svec F: Organic polymer monoliths as stationary phases for capillary HPLC. Journal of Separation Science 2004; 27(17-18): 1419-1430.
- Tanaka N, Kobayashi H, Nakanishi K, Minakuchi H, Ishizuka N: Monolithic LC columns. Analytical Chemistry 2001; 73(15): 420A-429A.
- Paull B, Nesterenko PN: New phases for rapid ion analysis. Euro. Pharm. Rev. 2005; 10(1): 47-51.
- Leinweber FC, Lubda D, Cabrera K, Tallarek U: Characterization of silica-based monoliths with bimodal pore size distribution. Analytical Chemistry 2002; 74(11): 2470-2477.
- Wu NJ, Dempsey J, Yehl PM, Dovletoglou A, Ellison D, Wyvratt J: Practical aspects of fast HPLC separations for pharmaceutical process development using monolithic columns. Analytica Chimica Acta 2004; 523(2): 149-156.
- Barbarin N, Mawhinney DB, Black R, Henion J: High-throughput selected reaction monitoring liquid chromatography-mass spectrometry determination of methylphenidate and its major metabolite, ritalinic acid, in rat plasma employing monolithic columns. Journal of Chromatography B-Analytical Technologies in the Biomedical and Life Sciences 2003; 783(1): 73-83.
- pb.merck.de/servlet/PB/menu/1209750/.
- www.phenomenex.com/Phen/Products/onyx/.
- Hatsis P, Lucy CA: Ultra-fast HPLC separation of common anions using a monolithic stationary phase. Analyst 2002; 127: 451-454.
- Hatsis P, Lucy CA: Improved sensitivity and characterization of high speed ion chromatography of inorganic anions. Anal. Chem. 2003; 75: 995-1001.
- Liu Y, Antonucci V, Shen Y, Vailaya A, Wu NJ: Practical applications of monolithic columns to pharmaceutical process development. J. Liq. Chromatogr. Rel. Tech. 2005; 28: 341-356.
- Ito K, Takayama Y, Makabe N, Mitsui R, Hirokawa T: Ion chromatography for determination of nitrite and nitrate in seawater using monolithic ODS columns. J. Chromatogr. A 2005; 1083: 63-67.
- Twohill E, Paull B: Zwitterionic ion chromatography using a dynamically coated column and mobile phase recycling. Journal of Chromatography A 2002; 973(1-2): 103-113.
- Pelletier S, Lucy CA: Achieving rapid low pressure ion chromatography separations on short monolithic columns. J. Chromatogr. A; in press.
- Sugrue W, Nesterenko PN, Paull B: Fast ion chromatography of inorganic anions and cations on a lysine bonded porous silica monolith. Journal of Chromatography A 2005; 1075(1-2): 167-175.
- Sugrue E, Nesterenko P, Paull B: Iminodiacetic acid functionalised monolithic silica chelating ion exchanger for rapid determination of alkaline earth metal ions in high ionic strength samples. Analyst 2003; 128(5): 417-420.
- Sugrue E, Nesterenko P, Paull B: Ion exchange properties of monolithic and particle type iminodiacetic acid modified silica. Journal of Separation Science 2004; 27(10-11): 921-930.
- Paull B, Nesterenko PN: New possibilities in ion chromatography using porous monolithic stationary-phase media. Trac-Trends in Analytical Chemistry 2005; 24(4): 295-303.
- www.metrohm.com/products/07/acc/columns.html.
- Urbansky ET: Assessment of perchlorate in fertilizers. Environmental Impact of Fertilizer on Soil and Water, vol 872, 2004; 16-31.
- Dasgupta PK, Martinelango PK, Jackson WA, et al.: The origin of naturally occurring perchlorate: The role of atmospheric processes. Environmental Science & Technology 2005; 39(6): 1569-1575.
- O’Riordain C, Nesterenko P, Paull B: Zwitterionic ion chromatography with carboxybetaine surfactant-coated particle packed and monolithic type columns. Journal of Chromatography A 2005; 1070(1-2): 71-78.
- Paull B, Riordain CO, Nesterenko PN: Double gradient ion chromatography on a short carboxybetaine coated monolithic anion exchanger. Chemical Communications 2005(2): 215-217.
- Nesterenko PN, Rybalko MA: The use of a continuous flow gradient for the separation of inorganic anions on a monolithic disk. Mendeleev Communications 2004(3): 121-122.
- Miller JM: Chromatography, Concepts and Contrasts, 2nd ed. Hoboken: Wiley Interscience, 2005.
- Zakaria P, Hutchinson JP, Avdalovic N, Liu Y, Haddad PR: Latex-coated polymeric monolithic ion-exchange stationary phases. 2. Micro-ion chromatography. Analytical Chemistry 2005; 77(2): 417-423.
- Hilder EF, Svec F, Frechet JMJ: Latex-functionalized monolithic columns for the separation of carbohydrates by micro anion-exchange chromatography. Journal of Chromatography A 2004; 1053(1-2): 101-106.
- Majors RE: Fast and Ultrafast HPLC on Sub-2-mm Porous Particles-Where Do We Go from Here? LC-GC North America 2005; 23: 1248-1255.
- Weiss J, Jensen D: Modern stationary phases for ion chromatography. Anal. Bioanal. Chem. 2003; 375: 81-98.
- Connolly D, Paull B: Fast separation of UV absorbing anions using ion-interaction chromatography. J. Chromatogr. A 2001; 917(1-2): 353-359.
- Connolly D, Paull B: Rapid determination of nitrate and nitrite in drinking water samples using ion-interaction liquid chromatography. Anal. Chim. Acta 2001; 441(1): 53-62.
- Connolly D, Paull B: Fast ion chromatography of common inorganic anions on a short ODS column permanently coated with didodecyldimethylammonium bromide. J. Chromatogr. A 2002; 953: 299-303.
- www.chem.agilent.com/Scripts/PDS.asp?lPage=1060.
- Pelletier S, Lucy CA: Ion Chromatography using Short Silica Columns. International Ion Chromatography Symposium, Montreal, Canada, September 18-21, 2005.
- https://chrombook.merck.de.




