SCTA for pharma
Posted: 20 July 2006 | | No comments yet
The determination of the key physical and chemical properties of a new material is essential. The melting point, glass transition temperature, the number and identification of the different phases it may have, and the temperatures at which they are formed are all of great value, not only in assessing its practical pharmaceutical potential but also as they can form the basis of many routine QC procedures.
The determination of the key physical and chemical properties of a new material is essential. The melting point, glass transition temperature, the number and identification of the different phases it may have, and the temperatures at which they are formed are all of great value, not only in assessing its practical pharmaceutical potential but also as they can form the basis of many routine QC procedures.
The determination of the key physical and chemical properties of a new material is essential. The melting point, glass transition temperature, the number and identification of the different phases it may have, and the temperatures at which they are formed are all of great value, not only in assessing its practical pharmaceutical potential but also as they can form the basis of many routine QC procedures.
Further information can be obtained from drying/desolvation, dehydration and decomposition studies. An excellent overview of the more common Thermal Analysis techniques and their many applications in the pharmaceutical industry has been given recently by Royall1. However, the need for the maximum amount of useful information constantly leads to a demand for both new types of information and increased sensitivity and resolution and this is where the new approach of Sample Controlled Thermal Analysis has great potential. As yet there are relatively few applications of SCTA in the field of pharmaceuticals and so this article will concentrate on the principles and illustrate the advantages of this new family of techniques using examples of the types of reaction which are widely studied in the pharmaceutical industry.
Conventional thermal analysis techniques are capable of further development to improve sensitivity and resolution and provide new types of information on materials. A significant new approach, developed independently by Rouquerol2,3, in France and the Pauliks4,5, in Hungary, was designed to address the limitations of conventional Thermal Analysis. Now termed ‘Sample Controlled Thermal Analysis, SCTA’, this has developed into a range of related techniques6 which confer many additional advantages in addition to minimising the problems alluded to below and some of which techniques are now available from several manufacturers.
Principles of SCTA
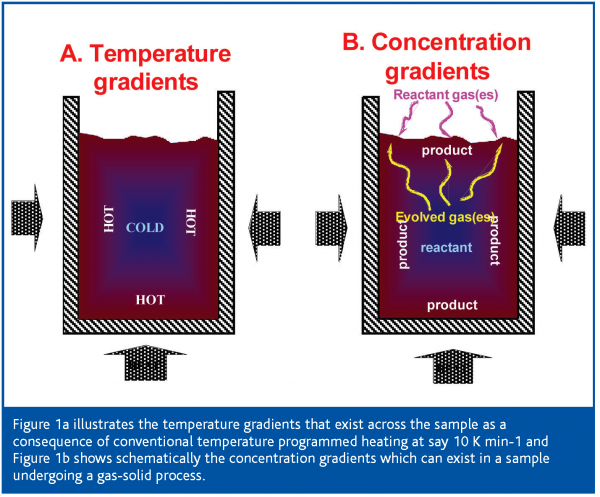
The essence of the SCTA approach is to recognise that in conventional thermal analysis, because heat is being applied to continually raise the temperature, significant temperature gradients will be produced throughout the sample (Figure 1a). These gradients arise because the exterior surfaces of the sample, through which the heat enters the bulk of the material being studied, are hotter than the interior. Thus any thermally induced process will be initiated at the surface of the sample and a complex reaction front will then travel to the centre of the sample which will gradually reach the temperature originally found at the exterior. Meanwhile the temperature of the exterior surface has continued to rise. Consider the effect this will have on a material with two closely spaced processes, for example dehydration followed by melting. The dehydration will start at the relatively hot external surface of the sample and will progress slowly inwards, depending on ability of the sample to conduct heat. Meanwhile, the exterior has already reached a higher temperature and has started to melt before the interior has completely dehydrated. As the two processes are occurring simultaneously in different parts of the sample at the same time the net effect is that of a dramatic reduction in the resolution of two processes. Of course, it is possible to minimise temperature gradients across a sample by using the lowest possible sample mass and keeping its form as a thin plate rather than a cylinder (as most heat is transferred thorough the gas-phase, at least at atmospheric pressure). Furthermore, it has long been known that the use of very slow heating rates improves resolution significantly, as a result of the consequent reduction in temperature gradients across the sample. In fact this is something of a simplification as one also has to consider the temperature gradients in the instrument itself, but these serve only to further complicate the situation. Another problem caused by the temperature gradients is that they cause uncertainty in the temperatures apparently observed.
Similarly, for any process involving a gas or vapour, such a solvent removal or oxidation, concentration gradients also will exist across the sample (Figure 1b). The result is that different vapour pressures will be found throughout the sample, possible leading to different reaction rates for reversible processes and, certainly, different reaction conditions throughout the material being studied. This again reduces resolution and causes significant errors in the measurement of the temperature of a process, especially for reversible processes such a drying or certain decomposition reactions. For instance, the thermal decomposition temperature of calcium carbonate can vary by several hundreds of degrees, depending on the pressure of the product CO2 over the sample.
The concentration gradients of product or reactant gas found during conventional heating regimes are shown in Figure 1b for both a reaction involving the evolution of a gas (e.g. drying) and the reaction of the sample with a gas, typically oxidation or reduction.
However, as SCTA controls the rate of reaction rather than the rate of heating, it is possible to minimise these gradient effects. As it happens, SCTA also brings other potential advantages and it has developed into an extensive family of techniques, under what has now become a generic title.
In essence, SCTA uses a sensor to follow the rate of the reaction. Commonly used systems measure reaction rate via the sample mass using a thermobalance (TG), the evolved or reacting gases using mass spectroscopy (MS) or dimensional changes with dilatometry. The system first detects the start of a process and then an automatic feedback loop comes into operation to control the rate of reaction of the sample. It is thus the sample itself which controls the rate of process it is undergoing, rather than a pre-determined heating rate, as in conventional Thermal Analysis. One of the most commonly used variants of SCTA is Constant Rate Thermal Analysis in which the system forces the sample to react at a constant rate. This rate can be set at any desired level but it is usually held at a very low value in order to minimise thermal and concentration gradients across the sample. The principles of SCTA are illustrated in Figure 2.
In the conventional TA experiment, the temperature rises linearly (1) and the parameter corresponding to the rate of reaction, in this case a product gas measured by MS, shows a sharp peak (2). It should be noted that the vertical axis is directly proportional to the reaction rate in the sample which is seen to vary extremely widely in conventional TA during the course of the experiment (2).
For the SCTA experiment, the temperature required to maintain the pre-set reaction rate is shown in red while the reaction rate is in green. Once the SCTA process has been induced by the initial linear temperature rise (3), the reaction rate increases (4) to the pre-set target level (5) at which point the feedback loop (6) begins to operate. The temperature is then made to follow whatever path is necessary (7) in order to keep the reaction rate (8) constant at the target level (5). Once the reaction being studied approaches completion, the reaction can no longer maintain the target rate and so decreases (9) and the temperature programme then returns to the set linear heating rate (10).
An example of the increase in resolution found using Sample Controlled Thermogravimetry (SCTG) to study a typical dehydration process is given in Figure 3. The dehydration of copper sulphate pentahydrate occurs in three steps, with the loss of two, two and one molecules of water respectively. The figure shows the results plotted against temperature and against time using conventional heating rates and the proprietary MaxRes® technique of Mettler Toledo. It can be seen that the SCTA approach produces significantly enhanced resolution in less time than a very slow conventional TG run.
The determination of free vs. bound water is not always straight forward as the two processes often overlap in conventional TG. However the use of High Resolution TG (TA Instruments) provides sufficient resolution to separate these two events.
Temperature resolved SCTA
Of course, once the SCTA system can control the rate of reaction at a constant level it becomes possible to control it in other ways also. For example, the relatively long run times of a Constant Rate experiment can be a disadvantage in a QC laboratory. To overcome this limitation it is possible to heat the sample quickly until the system detects a reaction or process and then to slow the reaction rate to a low level so that it can be completed before the next event is found. If the system then detects the end of this process, it automatically and dynamically increases the heating rate until the next reaction is detected where, once again, the heating rate is reduced to a low level to aid resolution7. This is known as operating in the temperature domain and gives rise to temperature resolved SCTA, which is by far the most common form of SCTA. It operates by monitoring the reaction rate and using its first, second and third differentials to detect the beginning and end of the processes being studied.
Time resolved SCTA
While Temperature Resolved SCTA is of great value, there are occasions when it is very useful to maximise the time between events so that, for example, the product of the first process can be removed for analysis before the sample reaches the temperature at which the second process commences. This can be achieved by using the control algorithms to cause the sample to be heated rapidly through events and very slowly between them resulting in high resolution in the time domain. In practice this means that when the TA curve is plotted against time the two peaks will be widely separated, even although when plotted against temperature the will appear to be close. The theoretical background to the use of complex reaction control methods is described elsewhere by Barnes et al.8.
SCTA can be used also to provide an insight into the mechanisms of reactions. Figure 49 shows the SCTG and conventional TG curves for the dehydration of strontium hydroxide octahydrate. The TG experiment appears to reveal a single stage mass loss, while the SCTG run shows that the sample is initially cooled slightly to about 20°C and held at this temperature until 7 H2O have been removed. The sample is then heated to 50°C when the remaining water is lost. The shape of the mass loss curve for the decomposition of the monohydrate is characteristic of that attributed to a mechanism involving nucleation and growth of nuclei.
Other SCTA methods include Stepwise Isothermal SCTA, Sorensen10,11, and Rate Perturbation techniques which allow the measurement of the energetics of the process being studied Rouquerol12. Criado13 introduced the concept of ‘Increasing Reaction Rate techniques and Charsley et al. have developed Sample Controlled thermomicroscopy techniques which will find great application in the study of solid-solid and solid-liquid transitions14.
Of necessity, such an extensive field cannot be covered fully in a short article and the reader is referred to the definitive reference work on SCTA edited by Rouquerol and Sorensen6 and the application of SCTA to catalysis15.
Acknowledgements
We gratefully acknowledge the kind permission of Mettler Toledo Ltd and TA Instruments Ltd to use Figures 3 and 4 and the EPSRC, ICI Catalco, ICI Synetix and Johnson Matthey for financial support.

References
- P. G. Royall, European Pharmacy Review, 4, 79, 2005
- J. Rouquerol, Bull. Soc. Chim. Fr. 31, 67, 1964
- J. Rouquerol, J. Thermochim. Acta, 144, 209, 1989
- L. Erden, F. Paulik, J. Paulik, J. Hungarian patent 152197, 1962
- F. Paulik, J. Paulik Thermochim Acta 23, 100, 1986
- O. T. Sorensen, J. Rouquerol (Eds) in Sample Controlled Thermal Analysis, Kluwer Academic, 2003
- G. M. B. Parkes, P. A. Barnes and E. L. Charsley, Analytical Chemistry, 2482, 71, 1999.
- G. M. B. Parkes, P. A. Barnes, E. L. Charsley, M. Reading and I. Abrahams, Thermochimica Acta, 39, 354, 2000.
- E. L. Charsley, J. J. Rooney, H. A. White, B. Berger & T. T. Griffiths, Proceedings 31st North American Thermal Analysis Society Conference, Albuquerque, New Mexico, Ed. M.Rich, NATAS, 133, 2003
- O. T. Sorenson, J. Thermal Analysis, 13, 429, 1978
- O. T. Sorenson, Thermochim. Acta 50, 163, 1980
- F. Rouquerol and J. Rouquerol, in H. G. Widemann (Eds.) Thermal Analysis, Vol. 1, Birkhauser, Basel, 373, 1972
- A. Ortega, L. A. Perez-Maqueda and J. M. Criado, J. Thermal Anal. 42, 551, 1994
- E. L. Charsley, C. Stewart, P. A. Barnes and G. M. B Parkes, J. Therm. Anal. Cal. 1087, 72, 2003.
- E. A. Fesenko, P. A. Barnes, G. M. B. Parkes, E. A. Dawson and M. J. Tiernan, Topics in Catalysis, 283, 19, 2002.




