Preparing for a new era in the rare disease sector
Posted: 28 February 2025 | Catherine Eckford (European Pharmaceutical Review), Christina Gkousgkouni (Sanofi) | No comments yet
To mark Rare Disease Day 2025, EPR interviewed Christina Gkousgkouni, Head of Rare Diseases for Central South Europe, Sanofi.


Rare disease research has surged over the past decade. When observing literature trends over the past twenty years, the amount of rare disease literature being published each year has more than tripled (Figure 1).1
Following the current trend, there has been a significant rise in the number of documents mentioning both artificial intelligence (AI) and rare diseases. For documents published in 2014 this number was just six; in 2024 it was 157 (Figure 2).1
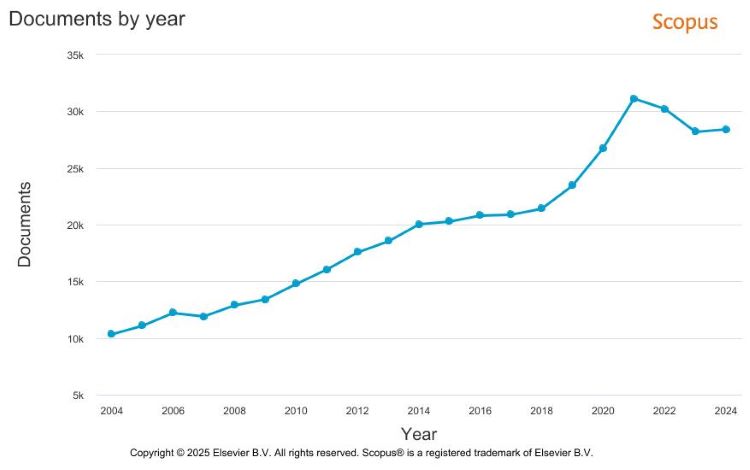

Figure 1: Documents by year. 2014-2024 (Credit: Elsevier BV – Scopus)
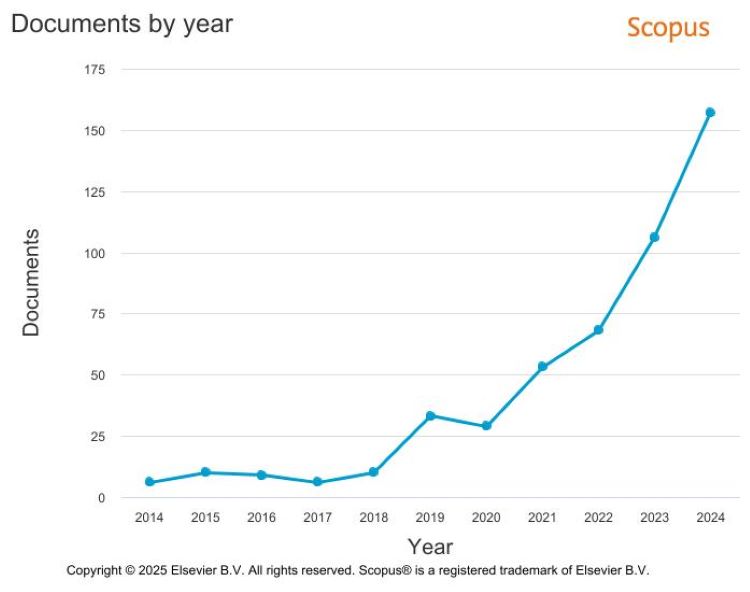

Figure 2: Documents by year. 2014-2024 (Credit: Elsevier BV – Scopus)
Regionally, the US is dominating rare disease research. Over the past decade, the country has increased the output of any other country by over fifty percent (Figure 3).1
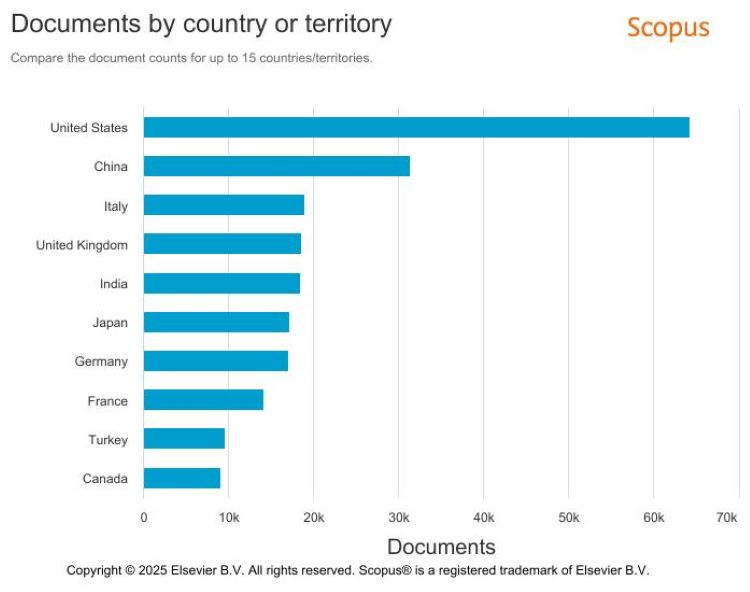

Figure 3: Documents by country or territory (Credit: Elsevier BV – Scopus)
The rare disease therapy landscape in the UK
In the UK however, challenges remain when it comes to providing rare disease patients with the medicines they need.
The Association of the British Pharmaceutical Industry (ABPI) recently reported on a survey of its member companies conducted in collaboration with the BioIndustry Association (BIA).
The UK Department of Health summarised the findings on the UK Rare Diseases Framework Board and Forum. One question enquired about the responding company’s rare disease pipeline and which proportion of the medicines were planned for launch in the UK. Data showed that out of the 18 companies that responded, 11 reported that they expected to launch less than 75 percent of their rare disease pipeline in the UK over the next five years, ABPI explained.
ABPI, Britain’s pharma industry body added: “Medicines policy over the past two decades has locked the UK medicines market into managed, long-term decline… Cost effectiveness thresholds in England are amongst the lowest in the world and have declined around 50 percent in real terms over the last twenty years.
“Unless we fix rocketing VPAG rates and improve recognition of value in NICE’s evaluation methods, UK rare disease patients face an increasing risk of not getting access to new medicines, which often represent their only treatment option”.
Rare disease clinical trials
While 300 million people worldwide live with rare diseases, they affect a small number of individuals, making it difficult to find eligible participants for clinical trials.2
To overcome the challenges of patient enrolment when there is widespread geographic distribution of eligible participants, key solution to address this includes optimising investigator site selection. Moreover, choosing trial sites based on proven enrolment capability means trials are more efficient and delays are limited.2
Regulatory compliance remains stringent in rare disease trials, despite limited patient numbers. However, sponsors can improve patient recruitment, optimise study design, and accelerate trial timelines while adhering to regulatory requirements.2
This can be achieved in several ways, for instance:
• Using advanced analytics, real-world data insights, and evidence-based investigator site selection strategies
• Using advanced trial design models to optimise study parameters to align with regulatory expectations while maintaining patient-centricity.
As each patient in a rare disease clinical trial has unique requirements, selecting standardised study endpoints and inclusion/exclusion criteria can be challenging.2
an adaptive, data-driven approach [is required to ensure] that rare disease trials are both scientifically robust and operationally feasible, ultimately leading to faster development and approval of life-changing therapies”
Research shows that rare disease Phase II protocols average 4.3 substantial amendments, nearly 65 percent more than the average for non-rare diseases. In Phase III, rare disease protocols average 3.8 substantial amendments, 19 percent higher than non-rare diseases.3
To minimise the needs for protocol amendments, understanding patient heterogeneity can enable more precise clinical trial design. Additionally, refining inclusion criteria and can be achieved more effectively through development of a detailed Digital Patient Profile.2
In summary, these approaches require an adaptive, data-driven approach, ensuring that rare disease trials are both scientifically robust and operationally feasible, ultimately leading to faster development and approval of life-changing therapies.2
With a pipeline including treatments for various rare anaemias and thrombocytopenias, alongside recent approvals, Sanofi is progressing with its efforts to address unmet needs in the rare disease treatment landscape.
Here, the company’s Head of Rare Diseases for Central South Europe, discusses major developments in the sector and what is needed to address current gaps. She highlights the importance of cross-collaboration, which technologies are shaping the future of rare disease therapies, and more.
What has been the biggest development in the rare disease treatment landscape over the past year and what could be done to accelerate this progress?
The past year has been a time of intense change in the treatment of rare diseases, with a key element being the rapid development of gene and RNA therapies. These innovative approaches allow healthcare professionals to precisely target the genetic mutations that cause many rare diseases.
At the same time, artificial intelligence (AI) is becoming an increasingly important tool in the development of targeted and enzyme-based therapies, resulting in better treatment outcomes and personalisation of therapies. The increased availability of reimbursed drugs should also not be overlooked, as a result of increased budgets and a better understanding of patients’ needs.
Particularly in Europe, it is equally important to increase investment in biotechnology and build stronger cooperation between science and industry for the benefit of European citizens”
From my perspective, the biggest development in rare diseases has been the tremendous increase of awareness around them! This required hard work by all the key stakeholders, including patient advocacy groups, the scientific community and the pharma industry, to increase visibility and understanding not only of the underlying disease mechanisms but also of the disease burden for patients and caregivers.
However, greater progress requires action on many fronts. First and foremost, patients suffering from rare diseases need more flexible regulations for clinical trials and more efficient drug approval processes.
Particularly in Europe, it is equally important to increase investment in biotechnology and build stronger cooperation between science and industry for the benefit of European citizens. It is also crucial to create public-private partnerships that will enable faster deployment of innovative therapies; we must highlight the continued unmet need for rare disease patients.
Most significant challenges in the rare disease sector? What solutions should pharma professionals focus on?
The most pressing challenge in our sector is undoubtedly bridging the gap between scientific breakthroughs and patient access, but this is particularly vital in rare diseases. Pharma professionals should focus on several key areas, including partnering with regulatory authorities and keeping focused on patient benefits.
There is a need to invest heavily in real-world evidence generation [to support rare disease sector]”
There is a need to invest heavily in real-world evidence generation. This means developing robust data collection methods post-approval to demonstrate the long-term value and effectiveness of novel therapies in everyday clinical practice.
By analysing vast datasets of genetic and molecular information, AI can uncover novel drug candidates or repurpose existing treatments for rare conditions. Machine learning models are already being applied to predict protein structures, facilitating the development of targeted therapies for conditions with limited treatment options.
Lastly, fostering collaborative ecosystems with regulators, payers and patient advocacy groups is crucial. We need to move towards value-based pricing models and innovative reimbursement strategies that ensure sustainable access to life-changing treatments. Science is making miracles in rare diseases treatment but society needs access to them; the health sector ecosystem must allow humanity to benefit from science.
What could improve collaboration between industry experts and regulators regarding rare disease therapies?
Early regulatory involvement in clinical trials and collaboration with research institutions would enhance adaptability. Expanding accelerated assessment programmes, digitalising approvals (eg, the clinical trials information system (CTIS)) and aligning EMA/FDA requirements will also streamline processes.
To ensure fruitful collaboration, we need education, transparency and ongoing dialogue. Training and educational programmes for both sides, focused on the latest developments, build common understanding”
Effective collaboration between industry experts and regulators in rare disease therapy development requires a unified European regulatory framework for simultaneous therapy registration across multiple markets.
Specifically, strengthening cooperation between Rare Disease Expert Centers (OECRs) at national and international levels can help create common guidelines. The important element is to have a common ground of understanding that both industry’s and regulators’ mission is to serve patients and develop mutual agreements that deliver a better quality of life to the population.
To ensure fruitful collaboration, we need education, transparency and ongoing dialogue. Training and educational programmes for both sides, focused on the latest developments, build common understanding. Joint educational initiatives for patient organisations bring regulatory processes closer.
Engaging patient organisations and industry stakeholders helps to identify clinical needs and drives innovation. Rapid consultations and initiatives like the Life Science Strategy promote knowledge sharing, strengthen research translation, and accelerate modern therapy availability.
In the next few years, what do you anticipate will shift the treatment landscape for rare disorders in Europe the most, and why?
Looking ahead, I believe several key factors will dramatically reshape the rare disease treatment landscape in Europe. Firstly, the continued acceleration of gene and cell therapies will be transformative. As these technologies advance, treatment will shift from symptom management to targeting root genetic causes.
several key factors will dramatically reshape the rare disease treatment landscape in Europe. Firstly, the continued acceleration of gene and cell therapies will be transformative”
Secondly, the integration of AI and big data will revolutionise diagnostics and drug development. AI-driven platforms will enable faster, more accurate diagnoses, crucial for rare diseases where time is often critical, and big data will speed up the discovery of new drug targets and repurposing therapies.
Thirdly, enhanced collaboration and data sharing across European countries will be vital. Initiatives like the European Rare Disease Platform5 and increased investment in cross-border clinical trials will facilitate the pooling of resources and expertise, leading to faster progress.
Finally, a growing focus on patient-centric care and regulatory flexibility will improve access to innovative therapies. Regulators are increasingly recognising the unique challenges of rare diseases and are adapting their frameworks to expedite approvals.
Anything else you would like to share?
I would like to emphasise the importance of sustained public awareness and education about rare diseases. While scientific advances are crucial, they can only truly benefit patients when combined with public understanding and support.
Progress in rare disease therapies is not just about scientific breakthroughs – it is a testament to the power of collaboration. From basic research to clinical trials and regulatory approvals, this is a collaborative effort that requires the dedication of scientists, clinicians, industry professionals and patient advocates.
About the interviewee


Christina began working in the pharmaceutical sector at Novartis, before joining Sanofi. A dedicated mentor and advocate for women’s leadership, Christina actively participates in initiatives like Women On Top and 100 Mentors. She holds a Master in Business Administration from Hellenic Open University and a Master of Science in Biology of Human Reproduction from the University of Thessaly.
References
1. [Internet] Elsevier Scopus. 2025. [cited 2025Feb]. Available from: https://www.elsevier.com/en-gb/products/scopus.
2. 5 Challenges of Delivering Effective Clinical Trials for Rare Diseases And Strategies for Overcoming Them. [Internet] Phesi. 2025. [cited 2025Feb]. Available from: https://www.phesi.com/.
3. Getz K. [Internet] Pharmaceutical Outsourcing. 2022. [cited 2025Feb]. Available from: https://www.pharmoutsourcing.com/Featured-Articles/584137-Doubling-Down-on-Protocol-Amendments-and-Deviations/#:~:text=In%20rare%20diseases%2C%20phase%20II,higher%20than%20non%2Drare%20diseases.
4. European Platform on Rare Disease Registration (EU RD Platform). [Internet] European Commission. [cited 2025Feb]. Available from: https://eu-rd-platform.jrc.ec.europa.eu/_en.
Related topics
Artificial Intelligence, Big Pharma, Biopharmaceuticals, Clinical Development, Clinical Trials, Drug Development, Drug Markets, Gene therapy, Industry Insight, Rare diseases, Regulation & Legislation, Research & Development (R&D), Technology, Therapeutics









