Cleanroom standards
Posted: 27 March 2007 | | No comments yet
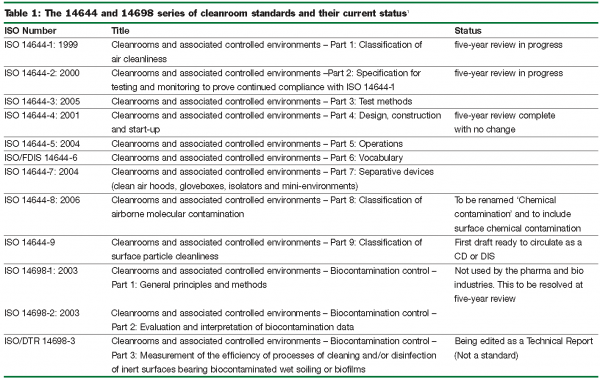
It is some years since the ISO 14644 and 14698 series of international cleanroom standards started taking over from national standards. Early parts are already undergoing their statutory five-year reviews; other parts have only recently been published and new parts are still being written.
It is some years since the ISO 14644 and 14698 series of international cleanroom standards started taking over from national standards. Early parts are already undergoing their statutory five-year reviews; other parts have only recently been published and new parts are still being written.
It is some years since the ISO 14644 and 14698 series of international cleanroom standards started taking over from national standards. Early parts are already undergoing their statutory five-year reviews; other parts have only recently been published and new parts are still being written.
These standards, prepared and approved by experts representing many nations and working in many different fields, are generic, leaving room for national or industry guidelines to cover specific applications. For example, the UK BSi will shortly publish PD 6609: 2007 – ‘Guide to in situ high efficiency filter leak testing’ to cover gaps in ISO 14644-3: 2005 – ‘Test methods,’ and the latest revision of Annex 1 of the EC GMP is eagerly awaited, with the expectation that it will be fully harmonised with ISO 14644-1: 1999 – Classification. The aim of this article is to update readers on all of these developments.
ISO 14644-1: 1999 – Part 1: Classification of air cleanliness
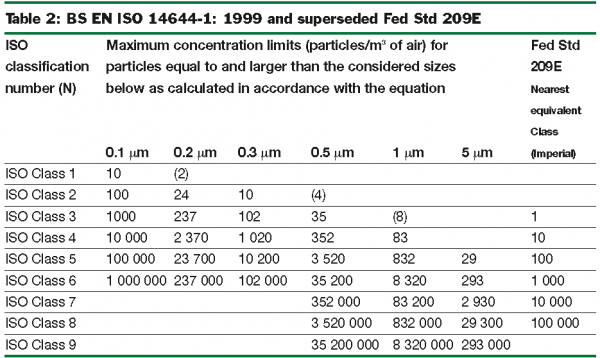
This replaces all existing national classifications including the widely used Federal Standard 209E (USA), now withdrawn. The five-year review will lead to a number of improvements. The ISO classification of particulate cleanliness will remain essentially the same but be based on the table, rather than the formula, which will be used for intermediate classes only. Some of the very low particle counts in the table may be removed as they require excessive sampling volumes. Table 2 shows these in brackets and compares the classification from ISO 14644-1 with that of US Federal Standard 209E. Class 100 of Federal Standard 209E has effectively become ISO Class 5.
The classification covers particles in the range 0.1 – 5.0 microns. Ultrafine particles <0.1 microns and macroparticles >5.0 microns are defined by U descriptors and M descriptors respectively. Particulate cleanliness is specified in one or more of three occupancy states, viz. ‘as-built’, ‘at-rest’, or ‘operational’. The end user will be interested in the ‘operational’ state, but verification of the ‘as-built’ and ‘at-rest’ classifications quickly highlight abnormalities.
The review will also improve the Annexes. The statistical treatment for sampling in Annex B will be simplified and the sequential sampling procedure in Annex F, clarified. Sequential sampling dramatically reduces sampling volumes and times when particle counts are significantly higher or lower than the class limits. The target date for publication of the revised ISO 14644-1 is January 2009.
ISO 14644-2: 2000 – Part 2: Specifications for testing and monitoring to prove continued compliance with ISO 14644-1
This sets out the normative maximum time interval between tests to demonstrate compliance with the particle concentration limits of ISO 14644-1. Not everyone agrees with the present frequencies. These will be reconsidered in the five-year review and there will be a better explanation of how increased monitoring allows for less frequent testing. An additional Annex to cover continuous (real-time) monitoring is under consideration. This is truly relevant to the requirements of EC GMP Annex 1. The target date for publication of the revised ISO 14644-2 is January 2009.
ISO 14644-3: 2005 – Part 3: Test methods
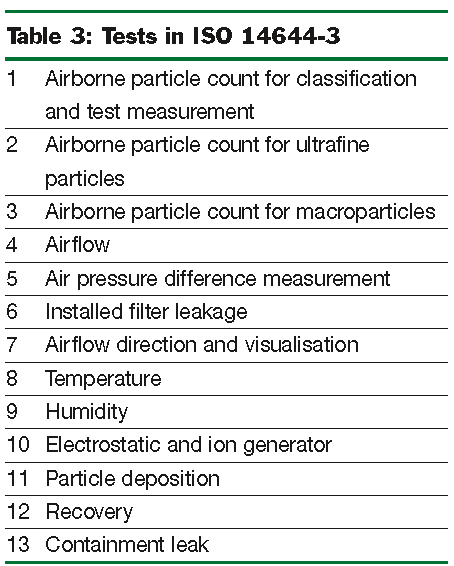
This lists most of the tests, mandatory and non-mandatory, that might be applied in a ‘clean installation’. It does not include specialised tests described in other parts, such as the leak tests in ISO 14644-7. Annex A is a checklist for planning test sequences, with cross references to test procedures in Annex B and test apparatus in Annex C. Table 3 lists all of the tests.
UK BSi document, PD (Published Document) 6609: 2007 – ‘Guide to in situ high efficiency filter leak testing’ will give supplementary guidance on installed filter leakage testing – see later.
ISO 14644-4: 2001 – Part 4: Design, construction and start-up
This specifies 16 requirements for the design and construction of a cleanroom to be agreed between purchaser and supplier. These include the required airborne particulate class, the critical environmental parameters and their specified set points, alert and action levels and the contamination control concept to be applied. A key term of the technology is defined thus: –
Unidirectional airflow: controlled airflow through the entire cross-section of a clean zone with a steady velocity and approximately parallel streamlines. NOTE: This type of airflow results in a directed transport of particles from the clean zone.
Examples of contamination control concepts are given in Annex A, with many useful diagrams. Despite the standard being generic, Annex B gives classification examples. For aseptic processing of healthcare products, ISO Class 5 is suggested, with unidirectional airflow and an average airflow velocity of >0.2 m/s. For microelectronics, ISO Classes 2 to 5 are suggested for different applications, all with unidirectional air flow and an average airflow velocity of 0.2 to 0.5 m/s. In the author’s experience, unidirectional air flow is more effective at 0.5 m/s.
Annex C describes a formal validation process. Annex D gives useful guidance on layout and airlocks and transfer hatches ‘to maintain pressure differential and integrity of the controlled space’. Further Annexes provide information on ‘Construction and materials’, ‘Environmental control’ and ‘Control of air cleanliness’. The final Annex has 13 checklists covering every aspect for consideration in the design of a cleanroom. The five-year review of ISO 14644-4 was completed in 2006 and the standard approved with no change.
ISO 14644-5: 2005 – Part 5: Operations
This specifies basic requirements for cleanroom operations in a checklist under six headings:
- Operational systems
- Cleanroom clothing
- Personnel
- Stationary [fixed] equipment
- Materials and portable equipment
- Cleanroom cleaning
Each of these is supported by an extensive ‘informative’ Annex with a daunting total of 38 references in the Bibliography! Annex A includes a requirement for a risk assessment to determine any relevant contamination control factors that may affect the products or processes in the cleanroom. Methods given for determining and managing these factors are:
a) HACCP (Hazard Analysis Critical Control Point)i
b) FMEA (Failure Mode Effects Analysis)ii,iii,
c) FTA (Fault Tree Analysis)iv
ISO/FDIS14644-6 – Part 6: Vocabulary
This is a ‘compendium of the terms and definitions used’ in all other parts of the 14644 and 14698 suite of standards. Publication will be in 2007. Where definitions differ between different parts (e.g. ‘particle’), the different definitions are listed. This will facilitate harmonisation when the respective parts come up for their five-year review.
ISO 14644-7: 2004 – Part 7: Separative devices (clean air hoods, gloveboxes, isolators and mini-environments)
The introduction explains the term “separative devices” which covers the range of application specific descriptions shown in brackets in the title. The normative sections cover requirements to be agreed between customer and supplier; aspects of design and construction for consideration; access devices, which include gloves and gauntlets, remote manipulation and robotic handling; transfer devices; siting and installing and testing and approval. Annex A describes the ‘Separation continuum concept’ which is a neat graphic explanation of how the ‘assurance of maintaining separation’ increases as the ‘separation means’ tightens from aerodynamic ‘unrestricted air overspill’ devices through to purely physical ‘high pressure integrity/low hourly leak rate enclosures’. Annex B gives guidance on ‘Air-handling systems and gas systems’, Annex C gives guidance on ‘Access devices’ and Annex D gives ‘Examples of transfer devices’. These examples derive from ‘Isolators for pharmaceutical applications’v, which was written by the UK Pharmaceutical Isolator Working Party. Annex E covers ‘Leak testing’ by reference to another ISO standardvii. Pressure integrity is defined in terms of hourly leak rate but takes no note of size. A method for estimating an acceptable hourly leak rate recognises the dilution effect of airflow in any space that would be contaminated by a leak. The Parjo test in Annex F is rarely used!
ISO 14644-8: 2006 – Part 8: Classification of airborne molecular contamination
This covers the classification of airborne molecular contamination (AMC) in terms of airborne concentrations of specific chemical species. Following publication, it was decided to include surface chemical contamination and change the title to ‘Chemical contamination’.
ISO CD/DIS 14644-9 – Part 9: Clean surfaces
Work has recently commenced on this and a draft is ready for issue as a CD or DIS. The scope covers only particulate contamination, as biocontamination is covered by ISO 14698-1 and -2, and surface chemical contamination has just been added to ISO 14644-8.
ISO 14698-1: 2003 – Biocontamination control – Part 1: General principles and methods
The ISI 14698 standards are part of the same work programme as the ISO 14644 standards. ISO 14698-1 establishes the principles and basic methodology for assessing and controlling biocontamination using one of the methods of risk assessment already referred to, with air, surfaces, textiles and liquids given as sources of biocontamination that can constitute a hazard. Guidance on the determination of biocontamination in these sources is given in the Annexes. Other Annexes give guidance on validating air samplers and on validating laundering processes. The five-year review, due in 2008, will address the limited acceptance of this standard in the pharmaceutical and biotechnology industries. Existing European biotechnology standards, prepared in support of European Directives on the use of genetically modified organisms and on the protection of workers from the risks relating to exposure to biological agents at work, cover similar ground.
ISO 14698-2: 2003 – Biocontamination control – Part 2: Evaluation and interpretation of biocontamination data
This gives guidance on ‘the evaluation of microbiological data and the estimation of results obtained from sampling for viable particles in risk zones for biocontamination control.’ The comments on ISO 14698-1 regarding the five-year review also apply here.
14698 Biocontamination control – Part 3: Measurement of the efficiency of processes of cleaning and/or disinfection of inert surfaces bearing biocontaminated wet soiling or biofilms
This will now be a Technical Report and not a standard.
PD 6609: 2007 – Guide to in situ high efficiency filter leak testing
Following the publication of BS EN ISO 1644-3: 2005: Test Methods, PD 6609 will be published by BSi in the early part of 2007. As with previous editions, it will supplement the most recent standards and also suggest templates for specifying filters and reporting tests. Because the in situ leak test differs from the filter manufacturers’ test, the in situ test should always be included in the specification when filters are ordered. The scope covers filters that can be face scanned, e.g. filters for cleanrooms and unidirectional airflow cabinets. A further standard is therefore needed for leak testing filters in configurations that cannot be readily scanned, such as exhaust filters, dual in line filters and cartridge filters, as used in separative devices (BS EN ISO 14644-7: 2004) and microbiological safety cabinets (BS 12469: 2000). A proposal for this has been put to BSi.
EC GMP Annex 1: Manufacture of Sterile Medicinal Products
Cleanroom practitioners have long held concerns about Annex 1, because the classification of air cleanliness, test methods and vocabulary are not harmonised with ISO 14644. In October 2005 EMeA (European Medicines Agency) published proposals for amendments to Annex 1 for public comments. Extensive comments were submitted by BSi (British Standards Institute), PHSS (Pharmaceutical and Healthcare Sciences Society) and ISPE (International Society for Pharmaceutical Engineering) amongst others. EMeA advise that there is now agreement in principle on all the issues but that the published document will not appear until the summer of 2007 at the earliest.
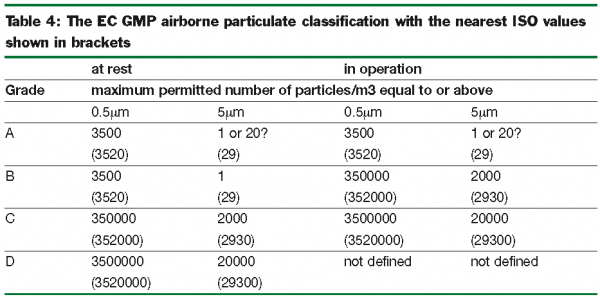
The most difficult issue has been the unharmonised classification of air cleanliness. Grades from EC GMP Grade A (the cleanest) down to Grade D are shown in Table 4 together with the nearest ISO values in brackets. For particles 5μm or greater, Grade A allows a maximum of 1/m3, with an ambiguous note in the EMeA proposals that ‘for reasons related to false counts associated with electronic noise, stray light, etc. the limit of 20/m3 could be considered.’
A major difficulty arises in testing. Annex 1 specifies a minimum sample volume of 1m3 to determine the particulate cleanliness classification of a given area irrespective of the particle size or specified classification, whereas ISO 14644, for statistical significance, requires a sufficient volume of air at each sampling location for the detection of a minimum of 20 particles of the largest considered particle size at the class limit of concentration for that particle size in the designated ISO class. By including 5mm particles (unlike the US FDA Guidance for Industryviii) Annex 1 stretches single sample volumes and times to impractical levels. The single sample volume to show that the number of particle/m3 is less than 1 would have to be 20 m3! Particle counters were developed for US Federal Standard 209 and sample at one cubic foot (0.0284 m3) per minute, so a 20 m3 sample would take a nonsensical 700 minutes. The single sample time for 0.5mm particles, where the class limit is 3500/m3, is 12 seconds. With continuous particle monitoring, provided a representative location can be agreed for the monitoring point, continuous monitoring of 0.5mm particles is perfectly practical. Again, this is not the case for 5μm particles. Full harmonisation would benefit manufacturers of particle counters, test engineers, designers and users of clean air equipment.
One further issue is the misuse of the term, ‘laminar air flow’. The correct term is ‘unidirectional air flow’ (see definition in ISO 14644-4 above). Laminar air flow has an entirely different meaning in all other fields of engineering. Likewise, the correct term for laminarity is uniformity of airflow.
Conclusion
Serious work has gone into the ISO 14644 series of cleanroom standards. These standards should now be fully accepted and adopted for all applications.




References
1. CD is Committee Draft, DIS Draft International Standard, FDIS Final Draft International Standard and DTR Draft Technical Report.
i PIERSON, M.D. and CORLETT, D.A.Jr.: HACCP Principles and applications. New York: Van Nostrand Rheinhold, 1992
ii IEC 60812:1985, Analysis techniques for system reliability – Procedure for failure mode and effects analysis (FMEA). Geneva, Switzerland: Commission Electrotechnique Internationale/International Electrotechnical Commission
iii Palady P.: FMEA, Failure modes and effect analysis. West Palm Beach, Florida: PT Publications, Inc., 1995
iv IEC 61025:1990, Fault tree analysis (FTA), Geneva, Switzerland: Commission Electrotechnique Internationale/International Electrotechnical Commission
v Isolators for pharmaceutical applications, ISBN 0 11 701829 5, HMSO, 1994, London UK
vi Pharmaceutical Isolators, A guide to their application, design and control, Midcalf BM, Phillips WM, Neiger JS, Coles TJ, ISBN 0 85369 573 3, Pharmaceutical Press, London, 2004
vii ISO 10648-2: 1994, Containment enclosures – Part 2: Classification according to leak tightness and associated checking methods.
viii Guidance for Industry: Sterile Drug Products Produced by Aseptic Processing – Current Good Manufacturing Practice, Food and Drug Administration (et al) September 2004, Pharmaceutical GMPs.








