Delivering long-acting oral treatments
Posted: 10 June 2024 | Kyle Haraldsen (Lyndra Therapeutics) | No comments yet
In this interview, Kyle Haraldsen, Chief Technology Officer of Lyndra Therapeutics, explores how the drug delivery landscape is evolving to increase focus on patient centricity and sustainability through development of long-acting, oral-delivery technologies.


How has the drug delivery landscape evolved in recent years?
Over the last few years, we have witnessed increasingly impressive innovations in the drug delivery landscape that have advanced our ability to prevent and treat disease. Everything from the mRNA technology that made the rapid development of COVID vaccines possible to antibody-drug conjugates enabling the binding of monoclonal antibodies to cancer receptors. One thing we haven’t seen a lot of, though, is improvements in the oral pill. Many of the pills we take today are not dissimilar to the aspirin pill from 100 years ago.
Some therapeutic areas, however, have seen an evolution towards developing longer acting medicines. There are generally two ways to do this – through chemistry of the drug molecule itself and through advanced delivery technology. As an industry, we tend to be more focused on the chemistry part, although there are some innovative things happening in delivery modalities thanks to advances in technology, too (eg, liposomal delivery, transdermal delivery, implantable devices).
For either focus, we need to make sure we’re creating things that patients actually want – being truly patient-centric. For example, research consistently shows that patients and caregivers prefer oral pills over medicine delivered via injection. It’s also human nature to be more comfortable with things that are already familiar to us.
This is why we’re focused on developing long-acting oral medicines at Lyndra. It’s a drug delivery method that patients know and like, and the potential benefits of long-acting medicines are clear: they can reduce patient and caregiver burden, increase medication adherence and, in turn, produce better patient outcomes.
Developing a long-acting delivery system at Lyndra
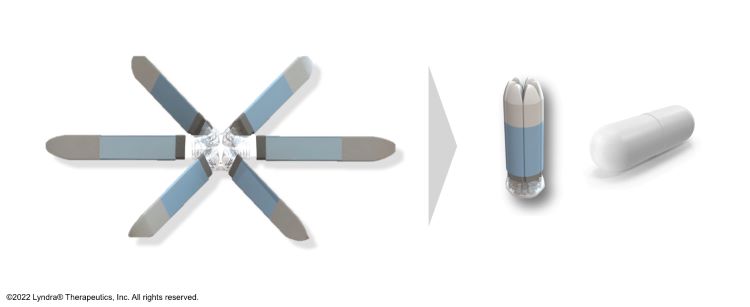

Lyndra has created a capsule that looks the same as the capsules people are used to taking, yet it has a proprietary coating that makes it easy to swallow and maintains its integrity until it reaches the acidic environment of the stomach. Once exposed to the stomach’s aqueous environment, the capsule dissolves and the arms within the capsule begin to elute the drug substance. Consistent elution of drug is made possible by suspending the drug in a polymer matrix. This controls the rate that stomach fluid enters into the drug arm to solubilise and elute the API. This technology enables the drug delivery platform to stay in the stomach for several days.
What are the top three trends in drug delivery?
There are several trends I could speak about, including an increased patient desire for self-administrable and low-cost options for injectable drug delivery systems and innovative uses of existing drug delivery systems/medications to target new therapeutic areas. There’s also a strengthening movement around sustainability as we innovate.
With lots of momentum in the space, here are three top trends I’ve been particularly focused on:
- Long acting gets longer acting: We’re creating a new category of medicines at Lyndra called “long-acting oral” therapies, so the growing demand from industry and patients here is a top priority for me. Pharmaceutical companies are taking notice and investing in the next generation of drug delivery technologies that will improve the dosing frequency of their medicines. Currently, we’re seeing this play out in the glucagon-like peptide-1 (GLP-1) space, as companies race against one another to create even longer-acting injectables and/or oral versions to compete for market share. In addition to helping patients, the creation of new long-acting drug delivery technologies can have major positive impacts for the industry. In some cases, creating longer-acting versions of a medicine may be an opportunity for patent extension. It could also bring new life to chemical entities that were previously not deemed viable medicines due to a burdensome dosing frequency.
- Oral drug delivery continues to be the ‘holy grail’: Companies manufacturing injectables are feeling pressure to offer oral options of their therapies to patients. For many years, the complexities of biologics manufacturing, the rapid degradation of medicines within the harsh environment of the gastrointestinal tract, and challenges with gastrointestinal permeability made this a challenging feat to accomplish. But recent advances in technology and processes are making it possible to deliver more medicines through oral pills. In some cases, companies that launched their drug to market as an injectable are now reinvesting some of those revenues to develop oral versions. It will be fascinating to see how companies continue to evolve by bringing injectables into oral therapeutic options, and the impact on patient preference and uptake.
- Patient centricity: Overall, the industry is focused on better meeting patient needs. This includes providing new routes for care and medication access with the growth of telehealth companies and the creation of medication delivery methods, packaging and support tools that aid their ease of use. We’ve seen this trend amplified in the GLP-1 space, with self-injection options offering patients a more convenient and discreet option for managing their medications. It is important we continue to consider and prioritise the things patients care about, including accessibility, affordability and environmental sustainability.
Please share an update on Lyndra’s remaining pivotal safety trial for the oral weekly risperidone (LYN-005) treatment for schizophrenia, planned for 2024.
In January 2024, we announced positive data from our pivotal Phase III study, comparing the pharmacokinetic profile of oral weekly risperidone (LYN-005) with immediate-release Risperdal administered daily to adults living with schizophrenia and schizoaffective disorder. Later this year, we will be advancing LYN‑005 into its remaining pivotal safety study and anticipate results will be announced in 2025, shortly followed by an NDA submission.
What are the two biggest challenges of manufacturing long-acting oral treatments?
- Longer feedback loops: The development of a long-acting pill means testing cycles also take longer. For example, if we’re doing dissolution testing for a pill that releases drug over a month, we really need at least 28 days to get results. Lyndra has created its own in-vitro testing technologies to help accelerate and streamline this process.
- Disruptive technology is novel: What Lyndra is doing with its LYNX® drug delivery platform (see box) is completely new. This adds complexity to the manufacturing process since we are also inventing new manufacturing systems, tools and processes. We do this while ensuring regulatory compliance throughout each step.
What two developments in long-acting oral treatments are you most excited about in the next five to 10 years?
As I noted earlier, we haven’t seen much development in long-acting orals over the last several decades, so any potential innovation in this space is incredibly exciting to me. I’m hoping to see more and more companies, and more and more investors, get as excited about the potential of long-acting oral therapies as we are. My sincere hope is that we see an infusion of investment in this space because I truly believe having access to long-acting oral therapies can be game changing for patients and our industry more broadly.
A few specific things that excite me are:
- The potential for long-acting oral therapies in polypharmacy. Lyndra’s LYNX platform has the capability to put different drugs in different arms. Pills taken individually can have different absorption profiles – but when combined in the platform, there could be a potential benefit of ensuring that the drugs are delivered optimally throughout the dosing period for a more synergistic effect. This needs more research, but I believe it could be a tremendous opportunity.
- I’m also optimistic that the platform will show our industry that we don’t need to give up on oral delivery of complex compounds – we don’t have to settle for injectables or other forms of delivery because a compound wouldn’t make a good daily pill. I believe the platform provides an opportunity to overcome some existing limitations and to open up new possibilities of long-acting oral drug delivery to improve patient lives and outcomes.
You can download the full Issue 2 2024 here.
About the interviewee


Issue
Related topics
Clinical Development, Drug Delivery Systems, Drug Development, Industry Insight, Research & Development (R&D), Therapeutics









