SFC-MS: advancements and applications in pharmaceutical quality control
Posted: 17 June 2024 | Cédric Hubert (University of Liège), Eric Ziemons (University of Liege Belgium), Hugues Jambo (University of Liège), Philippe Hubert (University of Liege Belgium) | 1 comment
Modern supercritical fluid chromatography systems coupled with mass spectrometry are empowering pharmaceutical quality control laboratories. Here, Hugues Jambo, Cédric Hubert, Éric Ziemons and Philippe Hubert from the Laboratory of Pharmaceutical Analytical Chemistry at the University of Liège discuss applications from impurity and nitrosamines detection to purity analysis.


Equipment from the Laboratory of Pharmaceutical Analytical Chemistry – University of Liège
Pharmaceutical quality control (QC) is an essential aspect of the pharmaceutical industry, ensuring that medicinal products meet the desired quality standards and comply with regulatory requirements. QC activities encompass a wide range of processes, from raw material inspection to finished product testing. Analytical techniques play a crucial role in QC, enabling the accurate identification, characterisation and quantification of various compounds in pharmaceutical products. These techniques help maintain product consistency, efficacy and safety, ultimately protecting patients’ wellbeing.
Supercritical fluid chromatography
Supercritical fluid chromatography (SFC) emerges as a versatile analytical technique increasingly used in the field of pharmaceutical QC. Unlike traditional liquid chromatography (LC) or gas chromatography (GC), SFC employs a supercritical fluid, typically carbon dioxide, as the mobile phase.
The resurgence of analytical SFC in the 2010s marked a significant milestone in chromatographic innovation. Instrument manufacturers such as Agilent, Waters, and later Shimadzu played pivotal roles in advancing SFC technology. By addressing the limitations of early instruments (such as lack of robustness, sensitivity and reproducibility), these companies have propelled SFC into mainstream analytical practice. Today, modern SFC systems provide high performance and reliability, empowering analysts with additional capabilities.
The adoption of SFC in pharmaceutical QC is underscored by its multiple advantages. When compared to (U)HPLC, SFC typically provides improved kinetic performance regarding efficiency even at high linear velocity.1 Additionally, SFC operates at lower pressure drops, permitting the use of very high flow rates.
These properties enable faster sample throughput, diminution of solvent consumption and increased productivity. Moreover, SFC has a broad analyte compatibility, permitting the analysis of diverse pharmaceutical compounds, from small molecules to peptides and chiral substances. Finally, SFC provides orthogonality to LC and GC, which enhances the analytical toolkit, allowing for more comprehensive compound characterisation.
Hyphenation of SFC to mass spectrometry
The coupling of SFC with mass spectrometry (SFC-MS) offers several opportunities for improving analysis in pharmaceutical QC. Chief among these is the significant improvement in sensitivity (compared to ultraviolet, for example) and the specificity this detection technique provides. The high sensitivity allows for the precise identification and quantification of analytes, even at trace levels. Furthermore, MS provides valuable structural information, enabling elucidation of compound structures and characterisation of complex mixtures.
Despite these advantages, the hyphenation of SFC to MS is not without challenges. One notable concern is the disparity in operating conditions between SFC and MS. While SFC typically operates at elevated pressures, MS ionisation occurs at atmospheric pressure in most common ion sources. This discrepancy can lead to rapid decompression of the supercritical fluid upon transfer to the MS source, resulting in analyte precipitation and loss of chromatographic efficiency. Controlling this decompression process is therefore essential to ensure optimal performance and reproducibility in SFC‑MS analyses.
To address these issues, various interfaces have been developed to facilitate seamless integration between the two techniques and are presented in Figure 1.2 These interfaces serve to maintain the integrity of the chromatographic separation while facilitating efficient transfer of analytes to the MS detector.
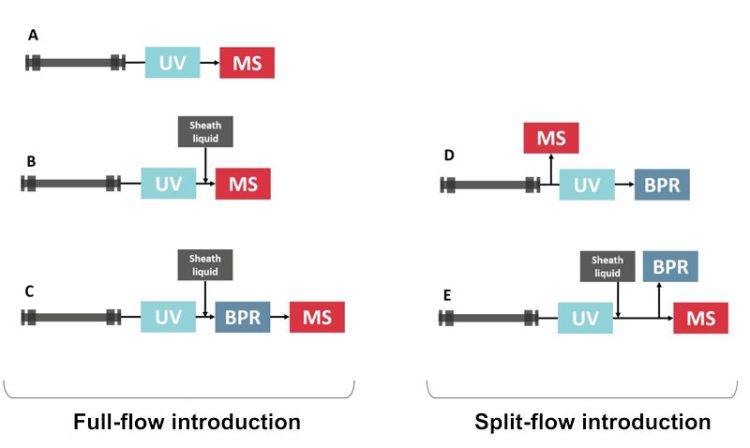

Figure 1: Schematic representations of various SFC-MS interfacing configurations.
(A) Direct coupling interface; (B) Pressure control fluid interface; (C) BPR and sheath pump with no splitter interface; (D) Pre-UV and BPR splitter without sheath pump interface and (E) Pre-BPR splitter with sheath pump interface. Adapted from reference 2 (https://doi.org/10.1016/j.jchromb.2020.122444).
The coupling strategies in SFC-MS can be categorised into two main approaches: full-flow introduction and split-flow introduction.
In the full-flow configuration, the entire effluent from the SFC column is directed to the MS inlet, providing potential to enhance sensitivity, particularly for mass‑flow sensitive ionisation techniques like atmospheric pressure chemical ionisation (APCI). The commercially available interface in this category is design C in Figure 1, also known as the “BPR and sheath pump with no splitter interface”. It involves the effluent passing through a back-pressure regulator (BPR) before entering the MS. Low-volume BPRs are crucial to minimise peak broadening, and a sheath liquid flow (also known as makeup flow) is often added to address post-BPR precipitation and improve MS signal. This design is utilised by Agilent and Shimadzu SFC systems.
In the split-flow configuration, however, only a minor portion of the effluent from the SFC column is diverted to the MS inlet, while the majority is directed through the BPR. This approach offers fewer constraints on the BPR design but may result in lower MS response, especially for mass-flow sensitive ionisation methods. Design E in Figure 1, also known as ‘MS split after UV with make-up pump’ interface, is the sole commercially available option in this category. It involves directing the column outlet first to a UV detector, then introducing a liquid organic make-up solvent before splitting the flow, with a minor fraction going to the MS and the majority to the BPR. This design is utilised by Waters and Agilent SFC systems.
Application of SFC-MS for pharmaceutical QC
The use of SFC-MS in pharmaceutical QC is a rapidly evolving field, with increasing mentions in literature. In this section, some recent applications will be presented.
Nitrosamines (NAs) impurities remain a prominent concern in the pharmaceutical industry, as they are classified as potential human carcinogens. Regulatory agencies have mandated all marketing authorisation holders to scrutinise their products for potential NAs impurities. In this context, Schmidtsdorff et al. developed SFC-MS/MS methods to detect NAs in both drug substances and products.3 In an initial investigation, sample analysis revealed NAs contaminations in ranitidine samples exceeding tolerance limits by tenfold. Subsequently, the authors applied these methods to analyse 16 NAs in 249 drug samples, detecting NAs in 2.0 percent of the cases.4
Today, modern SFC systems provide high performance and reliability, empowering analysts with additional capabilities
Continuing in the realm of impurities analysis, SFC-MS has also been used for the determination of seven vitamin D3 impurities in finished oily drug products.5 Two esters of vitamin D3 and five non-esters, including four isobaric compounds to vitamin D3, were targeted as impurities. Chromatograms obtained for their analysis are shown in Figure 2. The suitability of the SFC-MS method was evaluated through two validation studies. The first followed the ICH Q2 guideline for impurity limit testing to ascertain method specificity and established the limit of detection (LOD) and limit of quantification (LOQ) at 0.2 and 0.5 percent, respectively, for ester impurities. In the second study, a comprehensive quantitative assessment was conducted for three non-ester impurities using a total error approach, thereby determining method validity through accuracy profiles. The validated method demonstrated consistent performance across impurity concentrations in the range of 0.1 – 2.0 percent, with estimated LODs ranging from 2 to 7 ng/mL.
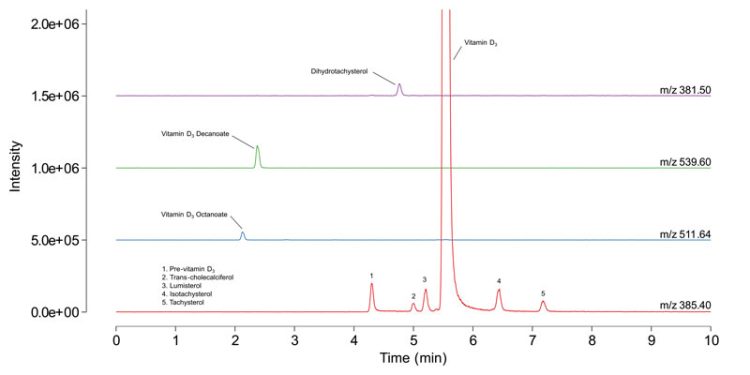

Figure 2: Chromatograms for a solution containing vitamin D3 impurities at 0.5 percent relative concentration (to the API) in n-heptane. Chromatograms were aquired on a Waters Acquity UPC2 coupled to a Waters SQD2 detector. Adapted from reference 5.
Another important aspect in pharmaceutical QC is determination of the chiral purity of drug substances and products. SFC is one of the most effective chromatographic techniques for these tasks and is routinely used in this domain – both the analytical and preparative scale. Hofstetter et al. developed and optimised an SFC-UV/MS method that permitted the chiral separation of ketamine and its metabolites in under eight minutes.6 The enantiopurity of pharmaceutical ketamine formulations could subsequently be tested, permitting to attest the quality of drug products.
The versatility of this technique for the analysis of a wide range of analytes has also been demonstrated in its use for the QC of therapeutic peptides. An SFC-UV/MS method for the fingerprinting of tyrothricin, a complex peptide antibiotic, was developed and shown to provide orthogonality and superior resolution compared to RPLC-based methods.7
Conclusion
SFC-MS stands as a valuable asset in the pharmaceutical industry’s ongoing quest for quality and safety. Modern instrumentation is robust, and the performance is adequate for the requirements of pharmaceutical QC. Some notable opportunities for this technique in this field include:
(i) throughput testing, permitting to enhance laboratory efficiency
(ii) replacement of normal-phase LC methods for a more sustainable solution
(iii) analytical workflow streamline due to the high versatility of this technique
(iv) integration in sustainable development policies in pharmaceutical companies.
It is also important to recognise the current limitations of this technique such as the need for improved instrumentation regarding extra-column variance, which is higher than UHPLC systems, knowledge gaps on some chromatographic behaviours, and the lack of training in this technique for the majority of routine QC laboratories. Ongoing research, development and implementation will undoubtedly permit to address these issues and strengthen the standing of this technique.
References
- Grand-Guillaume Perrenoud A, Veuthey JL, Guillarme D. Comparison of ultra-high performance supercritical fluid chromatography and ultra-high performance liquid chromatography for the analysis of pharmaceutical compounds. Journal of Chromatography A. 2012;1266:158–67.
- Van De Velde B, Guillarme D, Kohler I. Supercritical fluid chromatography – Mass spectrometry in metabolomics: Past, present, and future perspectives. Journal of Chromatography B. 2020 Dec;1161:122444.
- Schmidtsdorff S, Neumann J, Schmidt AH, Parr MK. Analytical lifecycle management for comprehensive and universal nitrosamine analysis in various pharmaceutical formulations by supercritical fluid chromatography. Journal of Pharmaceutical and Biomedical Analysis. 2021 Apr;197:113960.
- Schmidtsdorff S, Neumann J, Schmidt AH, Parr MK. Prevalence of nitrosamine contaminants in drug samples: Has the crisis been overcome? Archiv der Pharmazie. 2023 Feb;356(2):2200484.
- Jambo H, Dispas A, Pérez‐Mayán L, et al. Comprehensive analysis of vitamin D3 impurities in oily drug products using SFC–MS. Drug Testing and Analysis. 2024, in press.
- Hofstetter RK, Potlitz F, Schulig L, et al. Subcritical Fluid Chromatography at Sub-Ambient Temperatures for the Chiral Resolution of Ketamine Metabolites with Rapid-Onset Antidepressant Effects. Molecules. 2019 May 19;24(10):1927.
- Neumann J, Schmidtsdorff S, et al. Application of sub‐/supercritical fluid chromatography for the fingerprinting of a complex therapeutic peptide. J of Separation Science. 2022 Aug;45(16):3095–104.
About the authors


Hugues obtained his PhD in Biomedical and Pharmaceutical Sciences from the University of Liège (ULiège) in 2023. His doctoral research focused on leveraging supercritical fluid chromatography hyphenated to mass spectrometry for pharmaceutical quality control.


Cédric is an associate professor specialising in risk-based approaches for method development using the analytical quality by design (AQbD) strategy, which integrates the validation step. He is a co-author of one of the top 50 most cited review articles in the validation domain, published in the Journal of Chromatography A.


Professor of Green Pharmaceutical Analytical Chemistry and Vice-Director of the Center for Interdisciplinary Research on Medicines (CIRM) at the University of Liège (ULiège), Eric’s research is primarily focused on vibrational spectroscopy and hyperspectral imaging for pharmaceutical and biomedical analysis. He is the author of more than 120 peer reviewed articles.


Philippe is Professor of Pharmaceutical Analytical Chemistry and vice dean of the Faculty of Medicine of the University of Liège (ULiège). He has published more than 450 peer reviewed articles. His research focuses on separation techniques, vibrational spectroscopy and analytical methods validation applied to pharmaceutical and biomedical analysis.










I found the article on SFC-MS really insightful! The advancements in pharmaceutical quality control are fascinating, especially how SFC systems enhance efficiency and precision. It’s exciting to see how technology is evolving in our industry!