Maintaining a spore-free environment in the cleanroom
Posted: 21 September 2007 | | No comments yet
The manufacture of sterile pharmaceutical products is governed in the European Union by the requirements of EU Good Manufacturing Practice for Medicinal Products. The GMP guide gives very specific details on the environmental and microbial requirements for aseptic processing. However, little or no guidance is given on how to create and maintain the correct level of microbial contamination in the aseptic suite. This article focuses on two important issues – creating and maintaining a spore-free environment and preventing spore contamination that may result from the use of disinfectants.
The manufacture of sterile pharmaceutical products is governed in the European Union by the requirements of EU Good Manufacturing Practice for Medicinal Products. The GMP guide gives very specific details on the environmental and microbial requirements for aseptic processing. However, little or no guidance is given on how to create and maintain the correct level of microbial contamination in the aseptic suite. This article focuses on two important issues – creating and maintaining a spore-free environment and preventing spore contamination that may result from the use of disinfectants.
The manufacture of sterile pharmaceutical products is governed in the European Union by the requirements of EU Good Manufacturing Practice for Medicinal Products. The GMP guide gives very specific details on the environmental and microbial requirements for aseptic processing. However, little or no guidance is given on how to create and maintain the correct level of microbial contamination in the aseptic suite. This article focuses on two important issues – creating and maintaining a spore-free environment and preventing spore contamination that may result from the use of disinfectants.
As a disinfectant manufacturer, Shield Medicare offers a useful perspective on the selection and use of disinfectants, as it has an in-depth knowledge of the products and also operates its own cleanrooms to the same cGMP standards as pharmaceutical manufacturers.
Control of bacterial spores
One of the most difficult requirements in a life science cleanroom is the control of bacterial spores. They can enter the cleanroom on people and on components at a surprisingly high rate. Research has shown that 40% of consumables as they are taken from stores are contaminated with bacterial spores1. The rest of the study showed that if the transfer disinfection procedure employed, comprised of spraying solely with alcohol, only 27.6% of the spores would be removed. The results of the different methods tested are shown in Table 1. Annexe 1 of Good Manufacturing Practice (GMP) states that “Transfer of materials into and out of the unit is one of the greatest potential source of contamination”2.
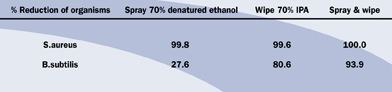

Table 1: Validation of Liquid Disinfection Techniques
The main problem with spores is their resistance to many of the standard disinfectants that are used, including alcohol, and their ability to lie dormant for long periods of time. Often a successful sporicide will have disadvantages, as it may be dangerous to humans, or equipment, or both. Hence the search for a non-hazardous but effective sporicide is very important.
Traditional disinfectants, such as alcohols, quaternary ammonium compounds, phenols and amphoteric surfactants are effective against bacteria in their vegetative state but useless against spores. Biocides that have sporicidal activity, including aldehydes, hypochlorites and hydrogen peroxide/peracetic acid blends are often toxic, irritant, corrosive, leave residues or need a long contact time.
An alternative sporicide is available, which is not as aggressive. It is an alcohol-free blend of a quaternary ammonium compound and stabilised chlorine dioxide. It is non-toxic, non-hazardous and non-corrosive, with a contact time of just five minutes and its efficacy against spores is adequate for almost all environments. Table 2 gives a comparison between different disinfectants.
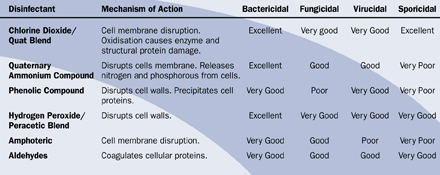

Table 2: Comparison of the Chemistry and Efficacy of Disinfection Agents
A key decision, therefore, is what level of sporicidal activity is required. A log 5 reduction in spores is achievable but this has other implications. For most cleanroom users a log 3 reduction is usually more than adequate.
Most disinfectants have efficacy data against standard tests, BS EN 1276 (bactericidal), BS EN 1650 (fungicidal), BS EN 13704:2002 (sporicidal) efficacy data. However, these standard tests are suspension tests and consideration should also be given to surface testing. Some biocides, due to the manner in which they work, will not perform well in suspension, but perform more than adequately on a surface test. Currently no surface test method is available for sporicidal efficacy. A test methodology can be found in BS EN 13697:2001 for surface testing against bacteria and fungi.
It is useful however to test under laboratory conditions the sporicidal effectiveness of a disinfectant on a surface. In the absence of an EN surface test for spores Shield Medicare has developed a Hard Surface Test3 based on BS EN 13697 and the AOAC carrier test. A log 3 reduction against spores on a surface is generally accepted as a pass criteria for this test.
Environmental isolates
Reported success with difficult environmental isolates, such as B.lichenformis and B.cereus, is invaluable. Another useful indicator is the experience of major reference sites – this should include successful action point use.
Another excellent source of data is from the commissioning of new cleanrooms, as this presents a significant challenge to a disinfectant. Table 3 shows the results from tests conducted by the QA department of a new sterile facility4. After only a builder’s clean, the environmental monitoring results identified gross contamination, including spores. The cleanroom was cleaned once using the new stabilised ClO2/Quat blend, a standard cleanroom mop and a double bucket system. The results after only one clean showed that almost all contamination was eradicated.


Table 3: Results from the Commissioning of a New Cleanroom
Specification
Whilst efficacy is important it is also crucial to consider the final specification of the disinfectant. The user must ensure it can be transferred easily into the cleanroom, that it meets the required standards for sterility, and that for a product contact area it is diluted with Water for Injection. Current GMP states that “disinfectants in Grade A and B cleanrooms should be sterile prior to use”2.
Similarly, a disinfectant may require a range of presentations and methods of application for different environments. Large areas may require concentrates for use with a mop and bucket system or a format suitable for fogging. Critical areas will need ready to use sprays or presaturated impregnated wipes. Presaturated wipes are especially useful in very critical areas, such as isolators and laminar flow cabinets, which may have areas where liquid can collect and is difficult to remove. Large, impregnated mop wipes also make short work of disinfecting cleanroom ceilings, walls and floors.
In all cases, the product must be supplied with essential batch documentation and certificates of sterility, irradiation and conformity where applicable. It is also essential to review the standard operating procedures to determine the implications that a particular biocide may have.
Health and safety
Alongside the effectiveness and usefulness of agents, a health and safety risk assessment is required as part of the validation process of a new sporicide. Some excellent disinfectants, particularly sporicides, are harmful and difficult to use in cleanrooms. The occupational exposure limits, the extra protective clothing or equipment required and the cost have to be considered for each agent. Input from personnel who have to use the disinfectants can also prove invaluable in the final selection.
Similarly, practical considerations such as on site storage and, increasingly, the waste disposal of chemicals or packaging materials, can have major implications resulting in additional costs.
Impact on the cleanroom environment
Many disinfectants, especially sporicides, are oxidising agents and the environmental impact of using these in a cleanroom containing expensive equipment has to be taken into account. A review of the available corrosion data on materials used in cleanroom, isolators and laminar flow cabinets should be a fundamental part of every validation programme.
Method of application
The application of the sporicide should be carried out to a validated procedure. The actual process of decontamination is the same for both cleaning and disinfection. Disinfection does not replace cleaning, as a heavily soiled surface may interfere with the effectiveness of a disinfectant. Cleaning should therefore usually be undertaken prior to disinfection, or combined with it. A separate cleaning stage is more likely to be needed if there has been a spillage of a product or sample. If a detergent is used, a rinsing cycle must be included to remove any residue. Any detergent residue remaining will have an adverse effect on any disinfectant used. Rinsing can be carried out with either Water for Injection (WFI), sterile purified or deionised water or sterile alcohol.
Due to the effect of biofilms, surface wiping is needed to assist in the removal of surface contamination. Wiping or moping should never be carried out in a circular motion as this causes the wipe/mop in its dirtiest state to be passed over an area which has just been cleaned. This point needs to be reinforced with operators, as a circular wiping pattern is the most comfortable and convenient according to Siegerman5.
The correct technique is to wipe/mop, towards you, in straight horizontal lines, each time overlapping the previous one by 10-25%. A contaminated wipe/mop should not be passed over an area that has just been wiped, unless in the case of a wipe it is folded and refolded to provide a clean surface. Usually quarterly folds are recommended but must be validated with each operator concerned, as a quarterly fold can lead to confusion as to which surfaces of the wipe have been used. In this case wipes folded in half should be used. Surface wiping, or mopping should be carried out from top to bottom, from back to front and from cleanest to dirtiest. The wipe or mop itself should be constructed from a low particulate material.
Preventing spore contamination from disinfectants
Maintaining the cleanroom integrity demands the safe delivery of the disinfectant: as mentioned previously, EU GMP Annexe 1 states that “Disinfectants and detergents used in Grade A and B areas should be sterile prior to use”2.
In most cases, disinfectants are delivered by either trigger sprays or aerosols. Trigger spray systems can offer significant advantages including:
- An adjustable nozzle so liquid can be dispensed as a jet or a spray, enabling thorough wetting of the surface and ensuring that disinfection procedures are effective
- They are environmentally friendly as they contain no propellants that require special disposal procedures
- All of the liquid can be dispensed from a trigger spray, so there is no wastage, making them a cost effective solution compared with an aerosol.
However, there is a significant potential drawback with some trigger sprays, as contaminated air can be drawn back into the bottle, compromising the sterility of the liquid. Validation work in the licensed pharmacy unit of the Queen Elizabeth Hospital in Birmingham, UK, identified that a trigger spray alcohol, used to spray items into an isolator, had been contaminated with fungal spores only 8 hours after opening6.
Improvements in the trigger spray system
Trigger spray systems are available which resolve this problem, providing a system with the benefits of a trigger spray whilst guaranteeing the integrity of the contents.
The new design operates as a closed system, preventing air being drawn back into the bottle and so eliminating the possible chance of contamination of the liquid. The liquid is held inside a medical grade bag; the function of the bottle is simply to enclose and protect the bag in use. All points of entry into the bag, including the dip-tube, are completely sealed, thereby creating the closed system.
In order to validate that the sterility of the liquid was not compromised during use, a series of tests were carried out on 1 litre bottles filled with sterile liquid nutrient7. Half the nutrient was sprayed out and the bottles left with their spray nozzles open in a Grade D cleanroom. Samples were removed at set time periods. The media was then tested at an independent laboratory to see if the contents had become contaminated. No growth was found in any of the samples, demonstrating that this design can protect the sterility of the contents for up to three months.
If all these areas are acted upon then it should be possible to maintain a spore free environment in the cleanroom.
References
- Validation of liquid transfer disinfection techniques for transfer of components into hospital pharmacy cleanrooms: Hospital Pharmacist Sept 2001: MG Cockcroft, D Hepworth, JC Rhodes, P Addison, AM Beaney
- MCA: Rules and Guidance for Pharmaceutical Manufacturers and Distributors 2002.
- Shield Medicare Technical Report Number TR0304R Test method for Shield Medicare Hard Surface Test for Sporicidal Activity
- Shield Medicare Technical Report Number TR0302R: Evaluation of the efficacy of Premier Klercide-CR Sterile Filtered Biocide B during the commissioning of a Medical Device cleanroom manufacturing facility
- Wiping Surfaces Clean: Advancing Applications in Contamination Control April 2003: Howard Siegerman
- Dr B Salvage: Contamination Control at your Fingertips: Pharmaceutical Manufacturing International, Autumn 1999
- Shield Medicare Technical Report Number TR9903R: An investigation into the in-use shelf life of Premier KlercideTM 70/30 Sterile Alcohol Sprays, Aug 2000








