The future direction of ASTM E55 Committee on manufacture of pharmaceutical products
Posted: 23 November 2007 | | No comments yet
ASTM Committee E55 formed in April of 2003 as a result of FDA’s GMPs for the 21st Century Initiative and the subsequent Guidance, “PAT – a framework for innovative pharmaceutical manufacturing and quality assurance.” Focusing on process understanding and flexible manufacturing, FDA encouraged the pharmaceutical industry to utilise the consensus standards approach to enable implementation of PAT principles into their manufacturing processes.
ASTM Committee E55 formed in April of 2003 as a result of FDA’s GMPs for the 21st Century Initiative and the subsequent Guidance, “PAT – a framework for innovative pharmaceutical manufacturing and quality assurance.” Focusing on process understanding and flexible manufacturing, FDA encouraged the pharmaceutical industry to utilise the consensus standards approach to enable implementation of PAT principles into their manufacturing processes.
The initial structure of E55 included three main subcommittees: E55.01, PAT System Management; E55.02, PAT System Implementation and Practice; and E55.91, Terminology. These three subcommittees focused on technical guidance documents, application standards and terminology, with the ultimate objective of standards to facilitate continuous quality assurance and real-time release. In December of 2005, subcommittee E55.03 was added, developing general pharmaceutical standards, which would facilitate PAT, and be broadly applicable beyond PAT. A detailed description of the initial structure, organisation and purpose of ASTM E55 can be found in the article, “Committee E55 – an update,” (see Reference 1).
In parallel with the development of Quality by Design concepts and the ICH Guidances Q8, Q9 and Q10, the ASTM E55 Executive Committee expanded its scope in 2006 from Pharmaceutical Application of PAT to the much broader Pharmaceutical Manufacturing. Specifically, the new scope of E55 now included “…development of standardised nomenclature and definitions of terms, recommended practices, guides, test methods, specifications, and performance standards for the manufacture of pharmaceutical products.”
In addition to expanding the scope, the Executive Committee also finalised a vision and mission aligned with the new scope.
Vision
ASTM E55 standards will be a major source of international standards that facilitate the design, development, understanding, evaluation, control and optimisation of modern pharmaceutical processes. These standards will support the community in establishing the most cost effective and transparent development and manufacture of consistent, high quality pharmaceutical products.
Mission statement:
The E55 Committee and supporting subcommittees will focus their efforts on standard terminology, guides, practices, test methods and specifications that directly support, add value and are cost effective for the pharmaceutical industry in developing and manufacturing high quality pharmaceutical products, using a scientific and risk-based approach. Standards, which simply codify existing practices, will be evaluated within E55. Only where there is a clear understanding on the added value and it is aligned to the E55 priorities will a work item be proposed.
Later in 2006, The Executive Committee of E55 made the decision to revisit its mission and vision, and evaluate its role in the standards setting process so as to complement the guidance and standards being developed by other organisations and regulatory bodies. That is, E55 Executive Committee planned to develop a Roadmap to the Future.
The ASTM E55 roadmap to the future
The preliminary roadmap
The initial roadmap was proposed in 2006, laying out the current standards and guidance under development in E55, along with potential future areas of need for additional standards in pharmaceutical manufacturing. This is depicted in Figure 1.
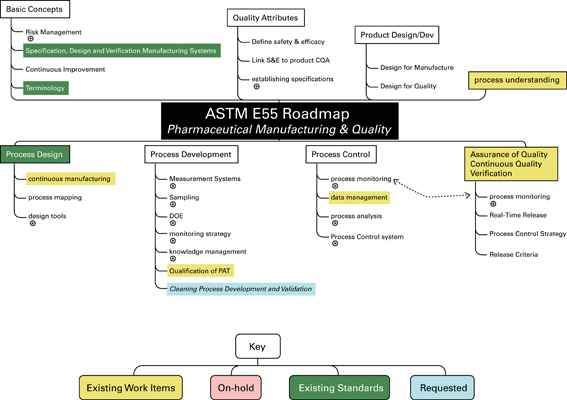

Figure 1
This first roadmap was used by the Executive Committee to reflect on the progress and direction of E55 since its beginning in 2003. As part of this process, the following questions were considered from the user-group, or customer, standpoint:
- What value am I getting from these standards?
- How can I use these standards?
- How do such different standards fit together?
- What do I tell the stakeholders in my company/organisation regarding ASTM E55 and the value of these standards to our business?
In addition to addressing these questions, the Committee evaluated the needs of the key stakeholders of the ASTM E55 process by considering the question, “What would make key stakeholders satisfied with the ASTM standards on pharmaceutical manufacturing?”
A summary of this discussion is shown in Table 1.
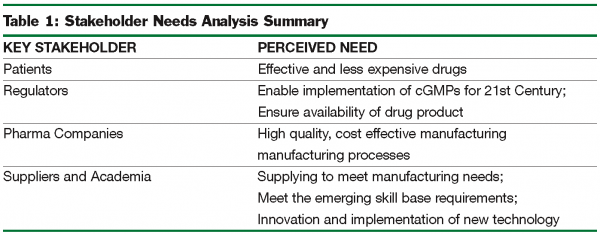

Table 1
The Committee looked at the results of this initial assessment, together with:
- The relatively slow pace of development of new standards
- The lack of clarity on the utility of ASTM standards for pharmaceutical manufacturing
- The low visibility of ASTM E55 both in the US and internationally
- Poor understanding of ASTM E55 among its key stakeholders.
It was clear that E55 needed to improve in a number of key areas in order to maintain its viability as a standards setting organisation for pharmaceutical manufacturing.
In October of 2007, the Executive Committee spent nearly two full days in a focused workshop in order to develop a strategy for re-establishing E55 as a key enabler for the pharmaceutical industry, through development and issuance of international consensus standards for pharmaceutical manufacturing. The initial roadmap first developed in 2006 was used as a basis for this strategy.
The workshop discussion concentrated on the following key components of the E55 standards setting process:
- Strategy
- Is the mission and vision still appropriate?
- Where do ASTM Standards fit into the current documentation hierarchy?
- Links to other organisations including ex-US, for example, ISPE, PDA, AAPS, EFPIA.
- Process
- How can we improve the standard-setting process?
- How can we measure our progress and continuously improve?
- People
- Are the right people in the right places leading ASTM E55?
- Do the stakeholders understand the role of ASTM E55?
- How do we get stakeholders to support the ASTM E55 effort?
- Structure
- Can we improve the effectiveness of the Executive Committee?
- Is the current meeting structure optimal for developing standards?
In this workshop, the Executive Committee planned to clarify how ASTM Standards are initiated and developed. Also of key importance was determining how they fit with other guidance; white papers and technical reports originating from multiple organisations and regulatory bodies, providing direction to industry on pharmaceutical manufacturing. The Committee also agreed to focus on the international aspects of ASTM E55, and the need to highlight the international role of its standards.
Source of standards for E55
The leadership of E55 recognised the need to stay connected with the many organisations and societies that develop pharmaceutical manufacturing guidance and standards. Building strong lines of communication and networking among organisations will result in optimising use of resources, focusing on the right standards, and ensuring alignment and consistency in standard development (Figure 2). The Executive Committee therefore agreed that ASTM E55 standards could come from multiple sources: organisations may request development of a consensus standard; individuals may request work on a specific standard; companies may petition E55 to write a standard. Based on the multiple sources of potential standards the Executive Committee also supported the concept that any proposal for a standard that fits within the mission and vision of E55 can be accepted as a work item. That is, the process should be driven by “need” for a standard regardless of the “level” of the standard (e.g. highly specific or very general). For example, the recently approved ASTM E55 New standard practice for qualification of basket and paddle dissolution apparatus is an excellent example of a very specific standard. By comparison, the New standard guide for the application of continuous quality verification to pharmaceutical manufacturing is an example of a more high level, general type of standard guidance. In addition to fitting with the mission and vision, the proposed standard must have some benefit to the user group, be based on sound science, and have sufficient support such that experts can by organised to write the standard in a timely manner.
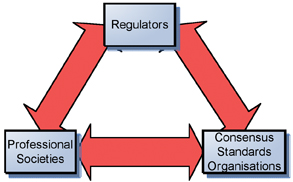

Figure 2: The information flow between various bodies giving rise to standards, guidance, white papers, and position papers.
E55 standards and the documentation hierarchy
As described in Figure 3, the regulations from Boards of Health occupy the top tier of a documentation hierarchy. These regulations define what companies must do to comply with the law. Sitting beneath the regulations are documents termed regulatory guidance and consensus standards (Tier 2). In contrast with the regulations, guidance does not have to be used, but ensures compliance if followed. Alternate methods can be used, but require justification. Similarly, consensus standards do not have to be used, but if they are used they are legally recognised as acceptable approaches globally. This demonstrates the power and usefulness of ASTM consensus standards. Finally, at the lower tier of the document hierarchy, are the position papers and technical reports, which do not have the force of regulations, standards or guidance, but do provide insight and information on a particular issue.
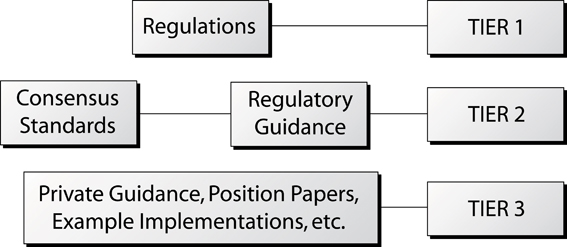

Figure 3: The relationships among the various types of guidance and standards documents.
International aspects of E55
Regarding international visibility, an EMEA observer has been attending recent E55 meetings. The Executive Committee is developing plans to foster international regulatory support for E55, as well as raise the visibility of ASTM with key stakeholders globally.
Conclusions
With these basic agreements on where standards originate and how they are accepted into E55 for development, the group established four sub teams to provide detailed proposals regarding four key aspects of E55 strategy: communications, vetting of standards, ways of working, and metrics.
- Communications: how do we interact both internally within E55 as well as externally with other organisations such as FDA, ICH, ISPE, EMEA. How do we raise the visibility of ASTM E55 internationally?
- Standard setting process: clarify and define the process for initiating, reviewing and approving standards.
- Ways of Working: revisit the meeting structure; improve the functioning of the executive committee; improve training of new ASTM members.
- Metrics: how can we track our progress and continuously improve?
The recommendations of each sub team will be finalised in the coming months, and an update on the roadmap progress will be shared with the membership of ASTM E55 in the November 2007 ASTM meeting in Tampa, as well as future meetings. The E55 Executive Committee is convinced that the Roadmap to the Future will ensure that key stakeholders view E55 as a value-added organisation, which supports manufacturing of pharmaceuticals with high quality in a cost effective manner, through development and issuance of relevant, usable consensus standards.
Approved E55 standards:
E2363-06a Standard terminology relating to process analytical technology in the pharmaceutical industry
1. Scope
1.1 This terminology covers process analytical technology in the pharmaceutical industry. Terms are defined as they are used relative to the PAT framework in the pharmaceutical industry. Terms that are generally understood and in common usage or adequately defined in other readily available references are not included except where particular delineation to process analytical technology may be more clearly stated.
1.2 This terminology is therefore intended to be selective of terms used generally in process analytical technology as it is applied in the pharmaceutical industry and published in a number of documents, such as those listed in the succeeding sections. The listing is also intended to define terms that appear prominently within other related ASTM standards and do not appear elsewhere.
1.3 The definitions are substantially identical to those published by the U.S. Food and Drug Administration and other authoritative bodies, such as ISO, IEC, ITU, and national standards organisations.
1.4 This terminology supplements current documents on terminology that concentrate on process analytical technology as it is applied in the pharmaceutical industry.
1.5 An increasing number of product designations and designations for chemical, physical, mechanical, analytical, and statistical tests and standards are coming into common usage in the literature, regulatory environment, and commerce associated with process analytical technology in the pharmaceutical industry. Section lists those documents referenced in this terminology.
E2474-06 Standard practice for pharmaceutical process design utilising process analytical technology
1. Scope
1.1 This practice covers process design, which is integral to process development as well as post-development process optimisation. It is focused on practical implementation and experimental development of process understanding.
1.2 The term process design as used in this practice can mean:
1.2.1 The activities to design a process (the process design), and/or
1.2.2 The outcome of this activity (the designed process).
1.3 The principles in this practice are applicable to both drug substance and drug product processes. For drug products, formulation development and process development are interrelated and therefore the process design will incorporate knowledge from the formulation development.
1.4 The principles in this practice apply during development of a new process or the improvement or redesign of an existing one, or both.
This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
E2500-07 Standard guide for specification, design, and verification of pharmaceutical and biopharmaceutical manufacturing systems and equipment
1. Scope
1.1 This guide is applicable to all elements of pharmaceutical and biopharmaceutical manufacturing systems including: facility equipment, process equipment, supporting utilities, associated process monitoring and control systems, and automation systems that have the potential to affect product quality and patient safety.
1.2 For brevity, these are referred to throughout the rest of this guide as manufacturing systems.
1.3 This guide may also be applied to laboratory, information, and medical device manufacturing systems.
1.4 This guide is applicable to both new and existing manufacturing systems. The approach may be used for the implementation of changes to existing systems, and their continuous improvement during operation.
1.5 This guide is applicable throughout the life-cycle of the manufacturing system from concept to retirement.
This standard does not address employee health and safety, environmental, or other non-GxP regulations. This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
E2503-07 Standard practice for qualification of basket and paddle dissolution apparatus
1. Scope
1.1 This practice covers the set-up and calibration of the paddle and basket dissolution apparatus.
1.2 Use of this practice may be applied to apparatus that have been modified to enable automatic dissolution testing (that is, a valve in the bottom of the vessel or sampling through the shaft).
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
Pat A. Picariello, J.D., CStd, Director, Developmental Operations, ASTM International
Reference
- Picariello, Pat. Committee E55 – an update. European Pharmaceutical Review, Issue 6, 2006.
About Steve Simmons
Head of Process Knowledge QbD, Wyeth Pharmaceuticals
Dr. Simmons is a graduate of Purdue University with a PhD in Industrial and Physical Pharmacy. He has worked for several pharmaceutical companies during his career of over 25 years, including Marion Labs, Lederle, and Aventis. Dr Simmons has had significant experience in many areas, including formulations research and quality operations. Currently he is Vice President of Pharma New Product Quality and Quality by Design within the Global Quality and Compliance organisation at Wyeth. Dr. Simmons is also the co-Chair of ASTM Committee E55.02.
Issue
Related topics
Good Manufacturing Practice (GMP), ICH guidelines, Process Analytical Technologies (PAT), Quality by Design (QbD)









