Regenerative potential: cell‑based therapies for heart failure
Posted: 27 July 2023 | Dr Ibon Garitaonandia (CellProthera) | No comments yet
Cell-based therapies have the potential to regenerate heart tissue as an alternative to heart transplants. Here, Dr Ibon Garitaonandia, Chief Scientific Officer at CellProthera, shares how CD34+ cells are demonstrating promising results in clinical studies.

Cardiovascular diseases are the leading cause of death globally with over 18.5 million fatalities per year, corresponding to over 30 percent of all deaths.1 Furthermore, there are 63.3 million people estimated to be living with heart failure,2 and the five-year survival rate is similar to cancer and stroke.3 Heart failure is characterised by fatigue and shortness of breath caused by a structural or functional cardiac abnormality that results in reduced cardiac output. Pharmacological therapies mainly address symptoms and unfortunately a significant number of patients do not respond adequately, progressing to congestive heart failure and eventually needing a heart transplant. There is a limited organ supply and only a few thousand heart transplants are performed per year worldwide with a waiting list that is ten times this amount and continues to grow every year. Additionally, there can be complications related to the continuous immunosuppression needed after heart transplantation, significantly limiting this therapeutic option.
More than 3,000 patients suffering from various cardiovascular diseases such as myocardial infarction, refractory angina and ischemic heart failure have received cell therapies in clinical trials
Cell-based therapies have emerged as a promising alternative with the potential to regenerate heart tissue. Thus far, more than 3,000 patients suffering from various cardiovascular diseases such as myocardial infarction, refractory angina and ischemic heart failure have received cell therapies in clinical trials. Despite these trials varying in design, they confirmed the feasibility and safety of the procedure. Unfortunately, the results of these clinical studies have been inconclusive with respect of efficacy, which could be explained by differences in the types of cells used, doses injected, routes of administration, timing of administration, clinical endpoints, and types of cardiac disease.4
Skeletal myoblasts were the first cell type tested in clinical trials for heart failure.5 Improvement in left ventricular ejection fraction was observed but unfortunately, episodes of sustained ventricular arrhythmia occurred in a small percentage of the population, likely due the differences in electrical conduction between the injected myoblasts and endogenous cardiomyocytes.6 Optimised approaches are now in the pipeline with human pluripotent stem cell-derived cardiomyocytes.
Bone marrow-derived mononuclear cells have been tested in almost 60 clinical trials. Meta‑analyses of these trials have shown that the therapy is safe and can lead to a reduction in incidences of death and recurrent myocardial infarction. However, when the clinical studies are considered individually, the results are inconclusive. This is probably because the CD34+ cell content within the injected cell population is small (0.5 to 1 percent).
Mesenchymal stem cells (MSCs) have also been extensively tested in cardiovascular clinical trials. MSCs have immunomodulatory and immunosuppressive properties but do not seem to differentiate into contracting cardiomyocytes. They secrete paracrine factors that enhance myocyte cell cycling and inhibit the formation of fibrosis. Results of the initial clinical studies have been promising but unfortunately late-stage clinical trials have failed to demonstrate improvement in cardiac function over placebo.
Clinical trials using CD34+ cells for heart failure
Only a few trials have tested CD34+ cells, but here I shall explain why they were the choice of CellProthera. After an acute myocardial infarction (AMI), the release of CD34+ cells by the bone marrow into the blood increases within the first few hours and continues for several days.
It has been demonstrated that the concentration of CD34+ cells in peripheral blood correlates significantly with the improvement in left ventricular ejection fraction. A week after the infarction, circulating levels of CD34+ cells return to normal, due to the secretion of chemokines by the lesion that recruit the cells to home to the ischemic area. This physiological response after AMI has a positive effect yet is unable to limit scar formation and compensate for the loss of infarcted cardiomyocytes. CellProthera is maximising this physiological response by expanding CD34+ cells in vitro and injecting them back into the patient’s heart.
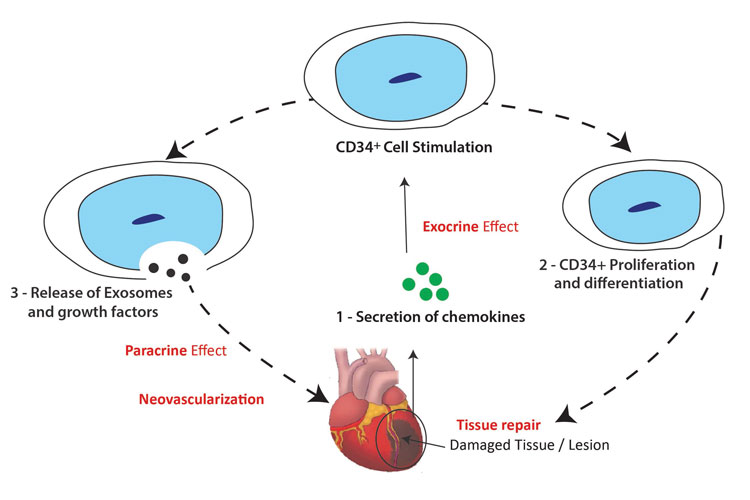

Figure 1: Diagram showing how CD34+ cells promote angiogenesis and revascularisation of damaged heart tissue.
CD34+ cells promote repair and regeneration of the heart lesion by secreting paracrine factors such as vascular endothelial growth factor and exosomes containing microRNAs (miRNAs) that induce angiogenesis and revascularisation of the damaged tissue. CD34+ secrete exosomes that contain miRNAs which enhance the proliferation of resident cardiomyocytes, prevent apoptosis, reduce fibrosis, and attenuate remodelling effects. CD34+ cells also contain endothelial progenitors that can differentiate into endothelial cells and promote the formation of new blood vessels in the scar tissue. We have demonstrated in pre-clinical studies that intramyocardial injection of CD34+ cells in rodent models leads to improved cardiac function and regeneration of heart tissue.7 Animals transplanted with expanded CD34+ cells have significantly higher cardiac output, increased ventricular compliance, and thicker ventricle walls than animals receiving placebo. We also showed that this therapy is safe with no toxicity, tumour formation, or biodistribution of the cells outside the heart.
In a pilot clinical study, the founder of CellProthera, Professor Philippe Hénon, showed that intramyocardial injection of autologous CD34+ cells in post-AMI patients is feasible, safe and leads to long-term improvement.8 Patients experienced significant improvements in left ventricular ejection fraction values at six, 12, 18 and 24 months after transplantation compared to baseline. Patients also had New York Heart Association‑grade improvement and myocardial structure regeneration and revascularisation with recovery of contractility in the previous akinetic area. All treated patients, except for one, survived for more than 12 years, compared to the 50 percent mortality generally observed five years after AMI. Even three patients who were initially recommended for heart transplantation no longer required it several years after the CD34+ cell injection. The only patient that did not respond to the therapy had suffered a myocardial infarction eight years prior to the cell injection and the lesion was already in an advanced stage of fibrosis and calcification at the time of injection, making it unsuitable for cell therapy.
After the successful results of this pilot study, CellProthera started a multi-centre, randomised, controlled Phase II clinical trial in severe AMI in multiple centres in France and the UK (NCT02669810). This clinical study has a target enrolment of 44 participants with 33 in the treatment group and 11 in the standard of care control group. The aim of the study is to evaluate the safety and preliminary efficacy of expanded autologous CD34+ cells (Protheracytes®). Interim results of this study are in line with those observed during the pilot study.
Manufacturing of autologous therapies
One of the main obstacles to overcome in autologous therapy is the variability of starting material and the costs of supply chain. The development of automated platforms with scale-out capacity is required to overcome these challenges.
Even though there have been many obstacles along the way, we believe that cell-based therapies for the treatment of cardiovascular diseases are bound for success
Protheracytes are manufactured under Good Manufacturing Practice (GMP) with our proprietary closed automated system, which is ISO 13485: 2016-certified, and with single-use cell culture kits. This process allows for the automatisation and standardisation of ProtheraCytes, which was registered by European Medicines Agency as an advanced therapeutic medicinal product (ATMP) in June 2013 (EMA/370710/2013). Even with this well‑established manufacturing process, we still face challenges when manufacturing batches because they sometimes do not meet release specifications. As this is an autologous therapy, there is a lot of patient-to-patient variability. To minimise this risk, we have optimised our cell culture media and cell purification method to improve production yield and product quality and introduced in-process quality control measures.
Allogeneic therapies using pluripotent cell sources that can be expanded indefinitely in culture allow for larger production and scale-up. However, they are prone to higher risk of genetic mutations which could potentially lead to tumours.9,10
Paving the way for CD34+ cell therapies
After the completion of our Phase II study, CellProthera intends to start a multicentric pivotal trial to gain market access in the treatment of post-MI patients.
Together with academic partners in Europe and in the USA, we are exploring the potential of ProtheraCytes® in different modalities and have already obtained promising pre-clinical data in other indications such as ischemic stroke with University of South Florida.
We believe that the success of our therapy is due to four key factors: timing of injection, cell type, cell number and injection route.4 The administration of the cells should be within two months after myocardial infarction, when chemokines are still present and scar tissue has not been formed. We believe that the CD34+ cells are the best therapeutic cell type because they promote the formation of blood vessels and the regeneration of ischemic lesions. Large numbers of cells should be administered as other clinical studies have shown that there is a dose‑dependent effect. And finally, the intramyocardial injection route leads to the highest cell retention as compared to the intracoronary and intravenous routes. Even though there have been many obstacles along the way, we believe that cell‑based therapies for the treatment of cardiovascular diseases are bound for success.
About the author
Ibon Garitaonandia, PhD, MBA is a seasoned executive who has advanced cell-based therapies from early discovery to clinical translation. He is currently the Chief Scientific Officer at CellProthera, a French clinical-stage biotechnology company developing stem cell- based therapies for cardiovascular and ischemic indications. Ibon was previously the Chief Science Officer at Richmond Research Institute (RRI) in London where he conducted clinical research in cardiac health, diabetes and several other conditions. Prior to joining RRI, he was Chief Scientific Officer at Histocell, a Spanish company developing cell-based therapies. Before that, he was the Director of Translational Research at California-based International Stem Cell Corporation. Ibon graduated in Chemistry from the University of the Basque Country and received his PhD in Biochemistry from the University of Florida.
References
- Terashvili M, Bosnjak ZJ. Stem cell therapies in cardiovascular disease. Journal of Cardiothoracic and Vascular Anesthesia. 2019;33(1):209–22.
- Campos de Carvalho AC, Kasai-Brunswick TH, Bastos Carvalho A. Cell-based therapies for heart failure. Frontiers in Pharmacology. 2021;12.
- Askoxylakis V, Thieke C, Pleger ST, et al. Long-term survival of cancer patients compared to heart failure and stroke: A systematic review. BMC Cancer. 2010;10(1).
- Hénon P. Key success factors for Regenerative Medicine in acquired heart diseases. Stem Cell Reviews and Reports. 2020;16(3):441–58.
- Menasché P, Hagège AA, Scorsin M, et al. Myoblast transplantation for heart failure. The Lancet. 2001;357(9252):279–80.
- Menasché P, Alfieri O, Janssens S, et al. The MYOBLAST autologous grafting in ischemic cardiomyopathy (magic) trial. Circulation. 2008;117(9):1189–200.
- Saucourt C, Vogt S, Merlin A, et al. Design and validation of an automated process for the expansion of peripheral blood-derived CD34+ cells for clinical use after myocardial infarction. Stem Cells Translational Medicine. 2019;8(8):822–32.
- Pasquet S, Sovalat H, Hénon P, et al. Pasquet, Design and Validation of an Automated Process for the Expansion of Peripheral Blood-Derived CD34+ Cells for Clinical Use After Myocardial Infarction. Cytotherapy. 2009;11:1002–15.
- Garitaonandia I, Amir H, Boscolo FS, et al. Increased risk of genetic and epigenetic instability in human embryonic stem cells associated with specific culture conditions. PLOS ONE. 2015;10(2).
- Merkle FT, Ghosh S, Kamitaki N, et al. Human pluripotent stem cells recurrently acquire and expand dominant negative p53 mutations [Internet]. Nature News. Nature Publishing Group; 2017 [Cited 2023Mar]. Available from: https://www.nature.com/articles/nature22312
Issue
Related topics
Clinical Trials, Drug Development, Regenerative Medicine, Research & Development (R&D), Stem Cells
Related organisations
CellProthera, European Medicines Agency (EMA), University of South Florida Health
Related drugs
Related people
Related diseases & conditions
acute myocardial infarction (AMI), cardiovascular disease (CVD), heart failure, ischemic stroke









